Epidemiology of cytomegalovirus infection and disease in hematopoietic stem cell transplant recipients in selected countries outside of North America and Europe: a systematic review
Clarisse M Machado1, Sung-Yeon Cho2, Monica Slavin3, Inderjeet Singh4, Anudeep Sandhu5, Dirk Demuth5, Depei Wu6.
1Institute of Tropical Medicine, University of São Paulo (IMT-FMUSP), São Paulo, Brazil; 2Seoul St. Mary’s Hospital, Seoul, Korea; 3Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne, Australia; 4Takeda Pharmaceuticals India Pvt Ltd, Gurgaon, India; 5Takeda International AG – Singapore Branch, Singapore, Singapore; 6Department of Hematology, First Affiliated Hospital of Soochow University, Suzhou, People's Republic of China
Background: Cytomegalovirus (CMV) is a highly prevalent herpes virus with a seroprevalence higher in South America, Africa, and Asia than in Europe and North America. In hematopoietic stem cell transplant recipients (HSCT), CMV infection is a common opportunistic infection and a major cause of morbidity and mortality. To better understand the epidemiology of CMV infection and disease post-HSCT in selected countries outside of Europe and North America, a systematic review was conducted.
Methods: Observational studies that included HSCT recipients (any age) from 15 selected countries in Asia-Pacific, Latin America, Russia and the Middle East were considered (search period: 01 Jan. 2011 – 21 Jul. 2021). Outcomes of interest were incidence, recurrence rates and risk factors of CMV infection and CMV disease, and CMV-related mortality at any time point. Indexed publications (Ovid® MEDLINE and Embase, Cochrane Database of Systematic Reviews and World Health Organization database Global Index Medicus) were searched, supplemented by pragmatic searches of the grey literature and snowballing of references lists. The protocol was registered in PROSPERO (CRD42020205559).
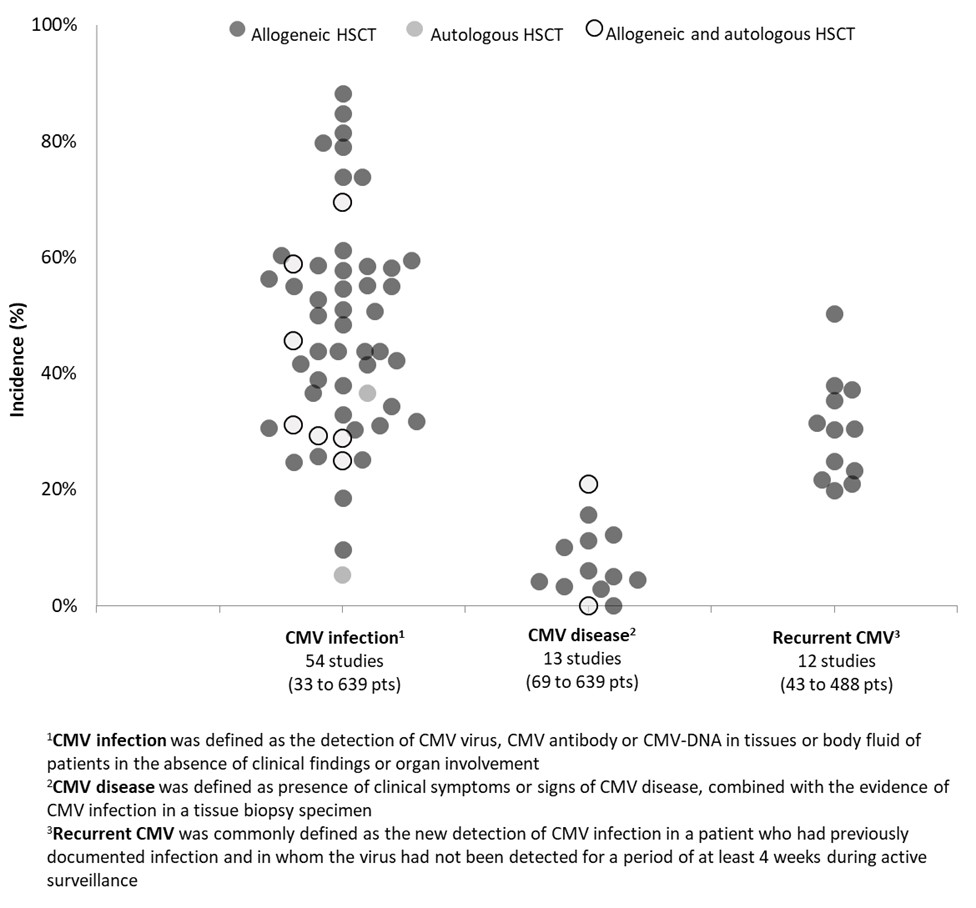
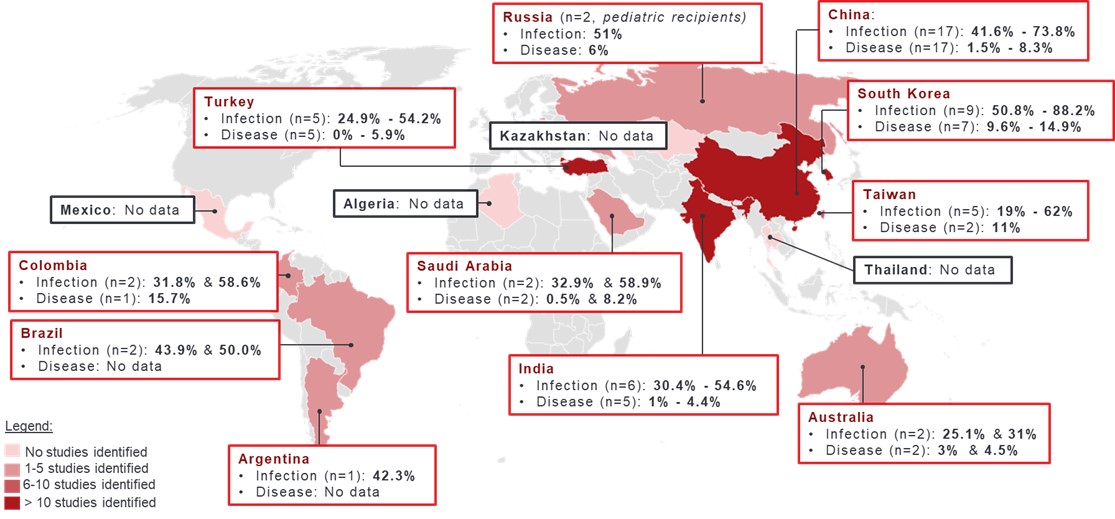
Results: A total of 63 studies (range: 33 to 10,206 patients), most conducted in adults (≥ 18 years; n=49 studies, 77.8%) and allogeneic HSCT recipients (n=53, 84.1%), were included in the review. In 43 of 54 studies reporting on incidence of CMV infection, within 1 year post-HSCT, estimates were uniformly distributed between 24.8% and 61.2% (Figure 1). Lower estimates were reported in autologous HSCT recipients (5.3%), and younger patients at the time of transplantation (9.3% in adults with a median age of 25 years and 18.6% in patients aged ≤20 years). Higher estimates (range: 69.4% to 88.2%) were found in 8 studies from China and South Korea, most with a follow-up period > 1 year (median: 27 to 54 months in 5 studies) (Figure 2). Incidence of CMV disease following HSCT was below 20%, with estimates uniformly distributed between 0% and 15.7% in allogeneic HSCT. According to 11 studies, reported rates of CMV recurrence ranged between 19.8% and 37.9%. Commonly reported risk factors for CMV infection or disease in HSCT recipients were high-risk CMV serostatus (R+) (hazard ratio [HR]: 2.6 and 3.7, odds ratio [OR]: 2.2 and 5.4), older age of recipients (HR for each additional year: 1.03-1.04), presence of acute or chronic graft versus host disease (HR: 1.1-2.5), haploidentical HSCT (HR: 2.7-6.4), and use of immunosuppressive agents (OR: 5.0-9.3). CMV-related mortality was reported in up to 10% of patients following HSCT (n=30 studies; period of assessment not reported).
Conclusion: Relatively high rates of CMV infection, CMV disease and CMV recurrence were reported post-HSCT, with CMV-related mortality observed in up to 1 in every 10 patients. High rates of CMV infection and disease post-HSCT may impact graft outcomes and increase disease burden in patients post-transplantation.
Literature retrieval, analysis and medical writing support were provided by Aurore Bergamasco, Camille Goyer, and Yola Moride of YolaRX Consultants and funded by Takeda International AG – Singapore Branch. CMM has received honoraria from Takeda and MSD (advisory board, speaker, educational events, meeting). S-YC has served as a consultant for Takeda Pharmaceutical, and has received research support and payment for lectures from Merck Sharp & Dohme, Pfizer and Gilead. MS has received grants from F2G, Gilead, Merck and personal fees from Gilead, Pfizer, Takeda and Roche for work outside of this research; IS is an employee of Takeda Pharmaceuticals India Pvt Ltd. AS and DD are employees of Takeda International AG – Singapore Branch and hold stock options. WD has no conflicts of interest to disclose. This review was funded by Takeda International AG – Singapore Branch.
