Pediatric donation after cardiac death (DCD) livers transplanted in adult recipients—appropriate utilization or missed opportunity for waitlisted children?
Christine Hwang1, Yash Kadakia1, Madhukar Patel1, Lucia de Gregorio1, Jigesh Shah1, Dev Desai1, Steven Hanish1, Parsia Vagefi1, Malcolm MacConmara2.
1Surgery, UT Southwestern Medical Center, Dallas, TX, United States; 2TransMedics, Amherst, MA, United States
Introduction: Donation after cardiac death (DCD) donors are increasingly recognized as an important source of donor livers. Pediatric DCD donor livers have been used successfully in pediatric recipients although this remains an uncommon occurrence. More frequently, pediatric DCD livers are used in adult recipients. We examined outcomes of pediatric DCD livers in pediatric and adult recipients.
Methods: The UNOS STAR file was accessed to determine all patients transplanted with a pediatric DCD liver between 2007-2020. A pediatric donor was defined as one who was under 18 years of age at time of donation; a pediatric recipient was defined as a patient under 18 years of age at the time of transplantation. Donor and recipient demographic data were examined, and a p value < 0.05 was considered to be significant.
Results: 473 pediatric DCD livers were utilized over the study period. 449 (95%) were transplanted into adult recipients (P-A), while 24 (5%) were transplanted into pediatric recipients (P-P). P-P donors were younger when compared to P-A donors (6.0 vs. 13.8 yrs, p<0.05), had a lower BMI (18.4 vs. 22.3, p<0.05), longer cold storage time (7.9 vs. 6.2 h, p<0.05), and a greater distance to transplant center (279.8 vs. 118.4 miles, p<0.05). Donor gender and ethnicity were similarly distributed. There were no significant differences between both groups in final donor AST levels, ALT levels or macrosteatosis content. Three P-P recipient experienced allograft loss secondary to hepatic artery thrombosis (HAT) (12.5%), and there was no allograft loss from biliary complications or primary nonfunction (PNF). In the P-A group, 8 allografts were lost due to HAT (1.8%), 5 from biliary complications (1.1%), and 6 as a result of PNF (1.3%). Allograft survival was significantly better in P-P when compared to P-A (p=0.02).
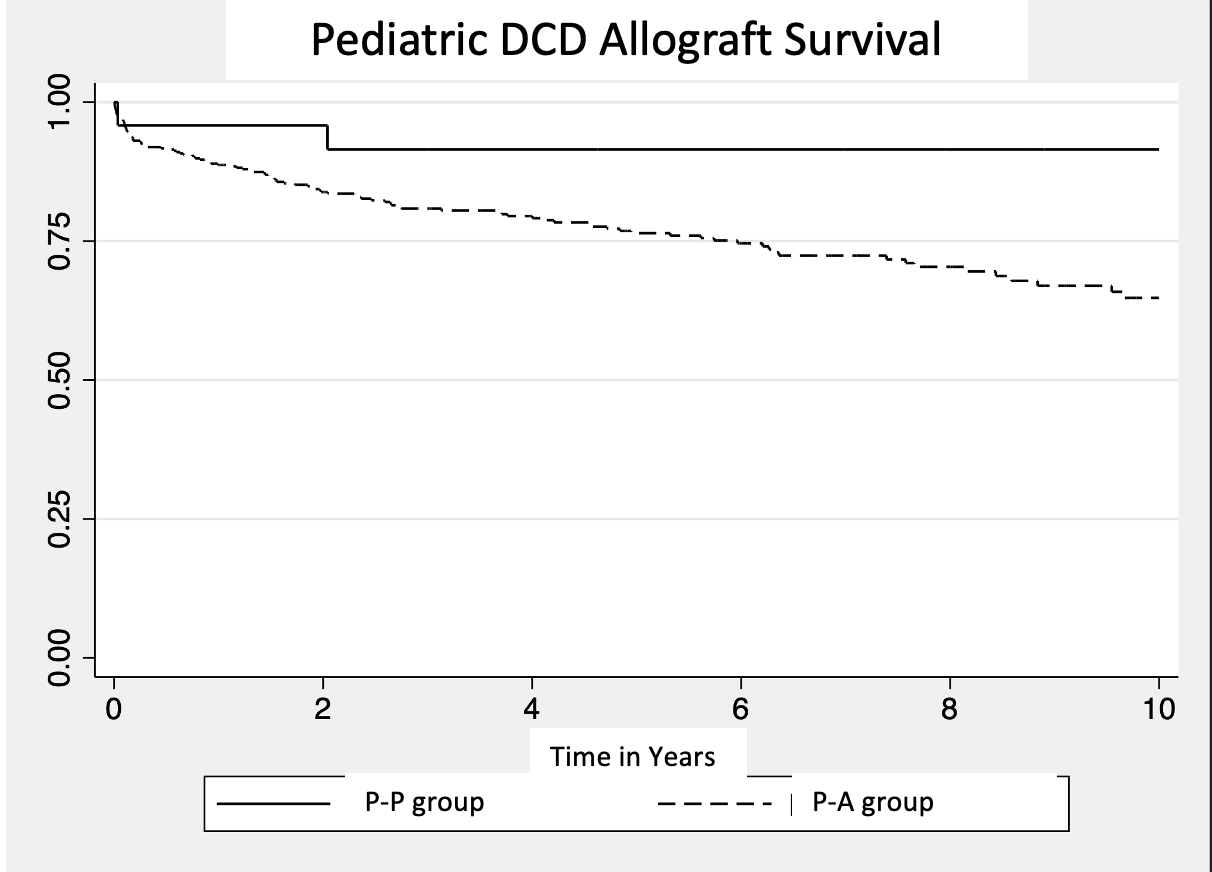
Conclusion: Utilization of pediatric DCD liver allografts is an uncommon occurrence; however, outcomes are excellent in pediatric and adult recipients. It is interesting to note that feared complications, such as biliary complications and primary nonfunction, were not present in the P-P cohort. Utilization of pediatric DCD allografts into appropriate pediatric recipients yields good outcomes and should be strongly considered.
