Barbara Kern, Germany has been granted the TTS International Transplantation Science Mentee-Mentor Awards
Donor dendritic cell depletion allows novel insights into the alloimmune response following murine composite tissue and skin transplantation
Barbara Kern1,2,3, Friederike Martin1, Joerg Mengwasser1, Anja Reutzel-Selke1, Muhammad-Imtiaz Ashraf1, Kirsten Fuehrer1, Steffen Lippert1, Dietrich Polenz1, Peter Tang1, Christian Witzel2, Igor Sauer1, Johann Pratschke1, Stefan Tullius4,5.
1Department of Surgery, Charité - Universitätsmedizin Berlin, Berlin, Germany; 2Department of Plastic and Reconstructive Surgery, Charité - Universitätsmedizin Berlin, Berlin, Germany; 3Berlin Institute of Health (BIH), Charité & Max-Delbrück-Centrum, Berlin, Germany; 4Division of Transplant Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States; 5Einstein BIH Visiting Fellow, Charité & Max-Delbrück-Centrum, Berlin, Germany
Background: Cell-based therapies in vascularized composite tissue allotransplantation (VCA) have demonstrated promising results with the potential to modify life-long conventional immunosuppression. Dendritic cells (DCs) promote pro-inflammatory, alloimmune responses. Of particular interest is the role of DCs as antigen-presenting cells and potential tolerogenic effects as immature DCs.
Material and Methods: Diphteria Toxin Receptor (DTR) transgenic or wild-type (WT) donor mice were used to deplete subsets of DCs or APCs respectively in a fully histoincompatible murine hind limb and skin transplant models; prior to procurement, all donors were either treated with diphteria toxin (DT; at -15 hours) or with clodronate (CL; 8 days prior to Tx). DBA/2J mice served as recipients. Study endpoint was on POD 6. Alloimmune response was assessed sequentially; graft survival, intragraft structural and inflammatory changes were tested serially.
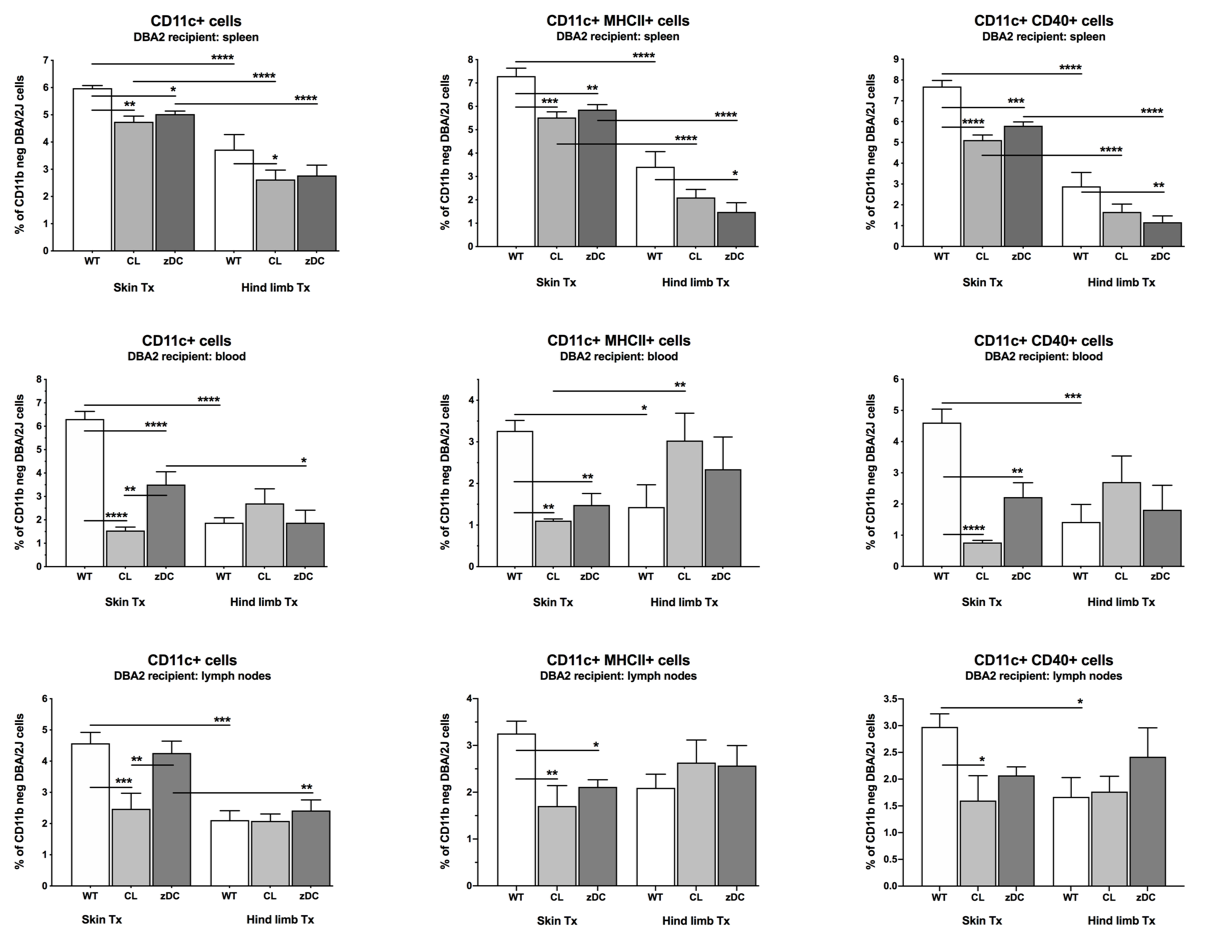
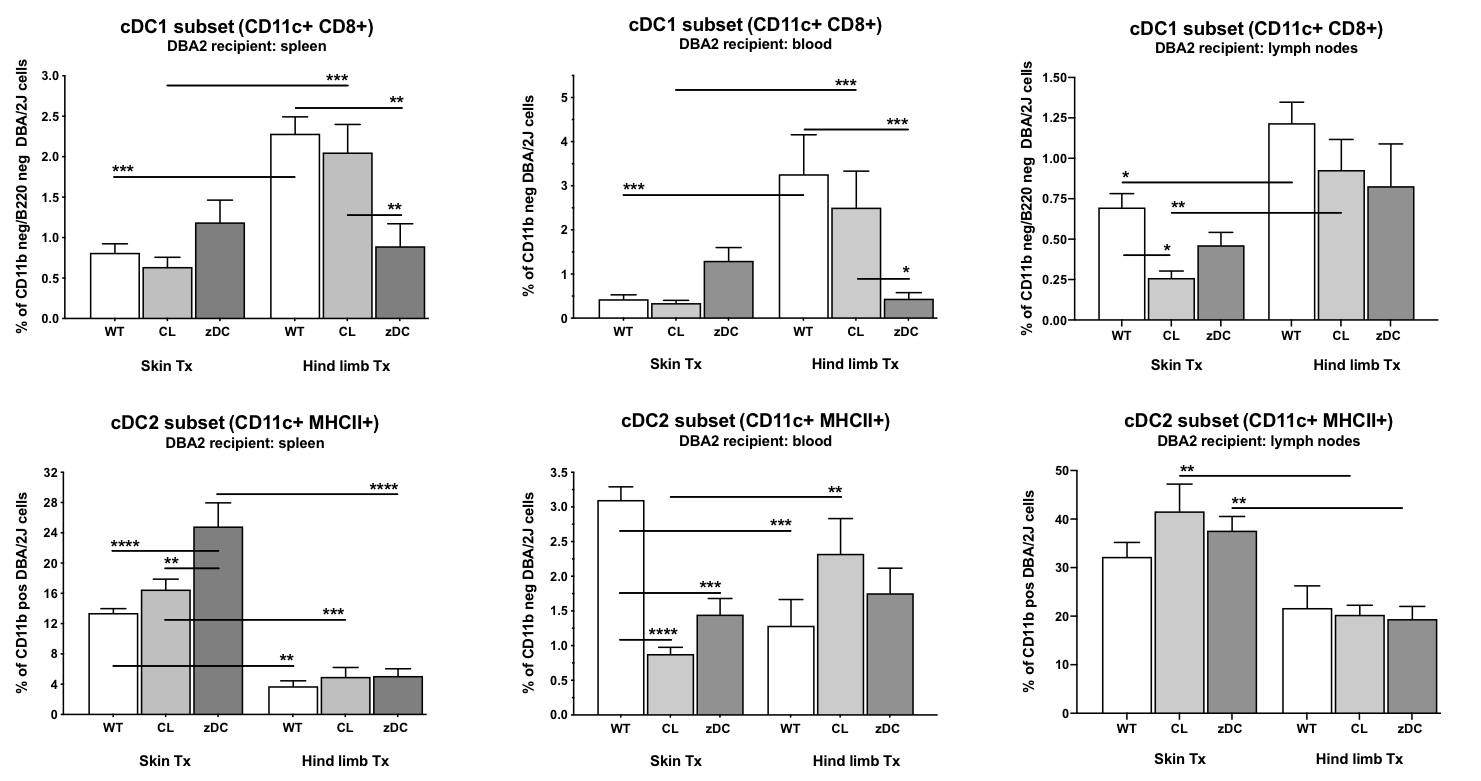
Results: The total number of dendritic cells (CD11b- CD11c+ DC) had markedly increased in recipients of skin WT grafts compared to recipients of VCA WT grafts (p<0.0001). The treatment with CL or DT significantly reduced the numbers of maturated and activated DC in the spleen, blood, and LN of mice receiving a skin graft, whereas numbers of CD11b-CD11c+MHCII+ and CD11b-CD11c+CD40+ DC were exclusively reduced in the spleen of VCA recipients after donor pretreatment with DT. cDC 1 subtypes were significantly higher in VCA WT vs. skin WT recipients (p<0.001). Donor pretreatment with DT decreased cDC1 counts only in recipients of VCA grafts (p<0.001).
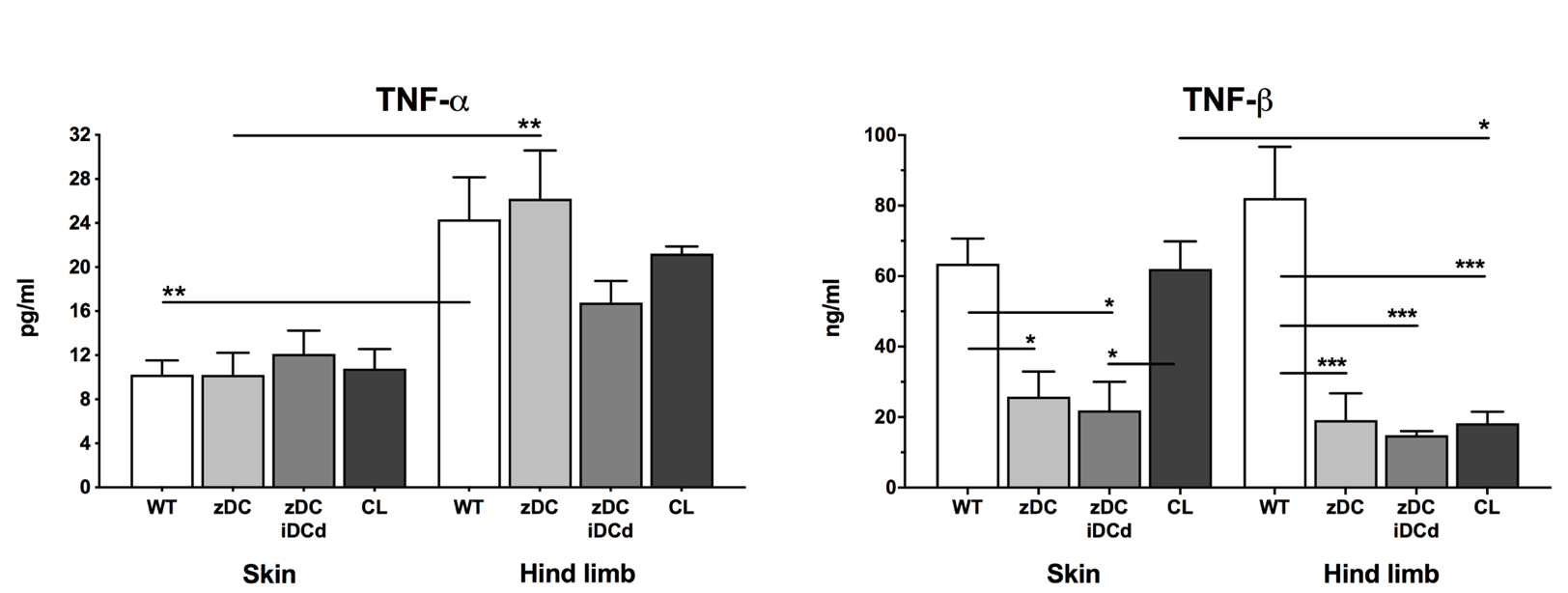
Luminex analysis revealed significantly higher TNF-alpha serum levels in the VCA WT vs. skin WT group (p<0.01). TNF-beta serum levels were significantly decreased in VCA depletion groups vs VCA WT group (p<0.001). Clinical signs of acute rejection among the VCA groups revealed higher rejection grades in the WT compared to the DC depletion group according to the BANFF criteria (grade III vs. grade II). Additional injection of immature DCs did not show any difference in allograft survival (p=ns).
Conclusion: This is to our knowledge the first systematic study delineating the role of mature and immature DCs tested across and skin transplants. Those data may help explaining split-tolerance phenomenon in VCA while providing a basic concept for the utilization of mature and immature DCs in modifying alloimmune responses in VCA transplants.
