Effect of CYP3A5 genotype-guided versus conventional initial dose of tacrolimus in children with kidney transplants
Ana Catalina Alvarez Elias1, Maria del Pilar Garcia-Roca1, Luis Velasquez-Jones2, Saul Valverde-Rosas2, Gustavo Varela-Fascinetto3, Mara Medeiros-Domingo1.
1Unidad de Investigación y Diagnóstico en Nefrologia y Metabolismo Mineral Oseo, Hospital Infantil de Mexico, Federico Gomez, Delegacion Cuahutemoc, , Mexico; 2Departamento de Nefrologia, Hospital Infantil de Mexico Federico Gomez, Delegacion Cuahutemoc, , Mexico; 3Cirugia de Trasplante, Hospital Infantil de Mexico Federico Gomez, Delegacion Cuahutemoc, , Mexico
Background: CYP3A5 tacrolimus polymorphisms predict its metabolization. We aimed to test the advantage of a genotype-guided starting dose after kidney transplantation to reduce the time to target drug levels and improve allograft surveillance.
Methods: We performed a single-center, open-label parallel group, randomised controlled trial in kidney recipients (0-18 y/o) at the Hospital Infantil de México, Federico Gómez, from Jan-2013 to Dec-2018. Patients were assigned to one of two groups. The genotype-guided group received the dose according to the CYP3A5 polymorphisms (AA1*1, AG1*3 [expressers], and GG3*3, [non-expressers]). The Conventional-dose group received standard of care. We followed them for 12 months registering time to target levels, estimated glomerular filtration rate (eGFR), rejection, and nephrotoxic events.
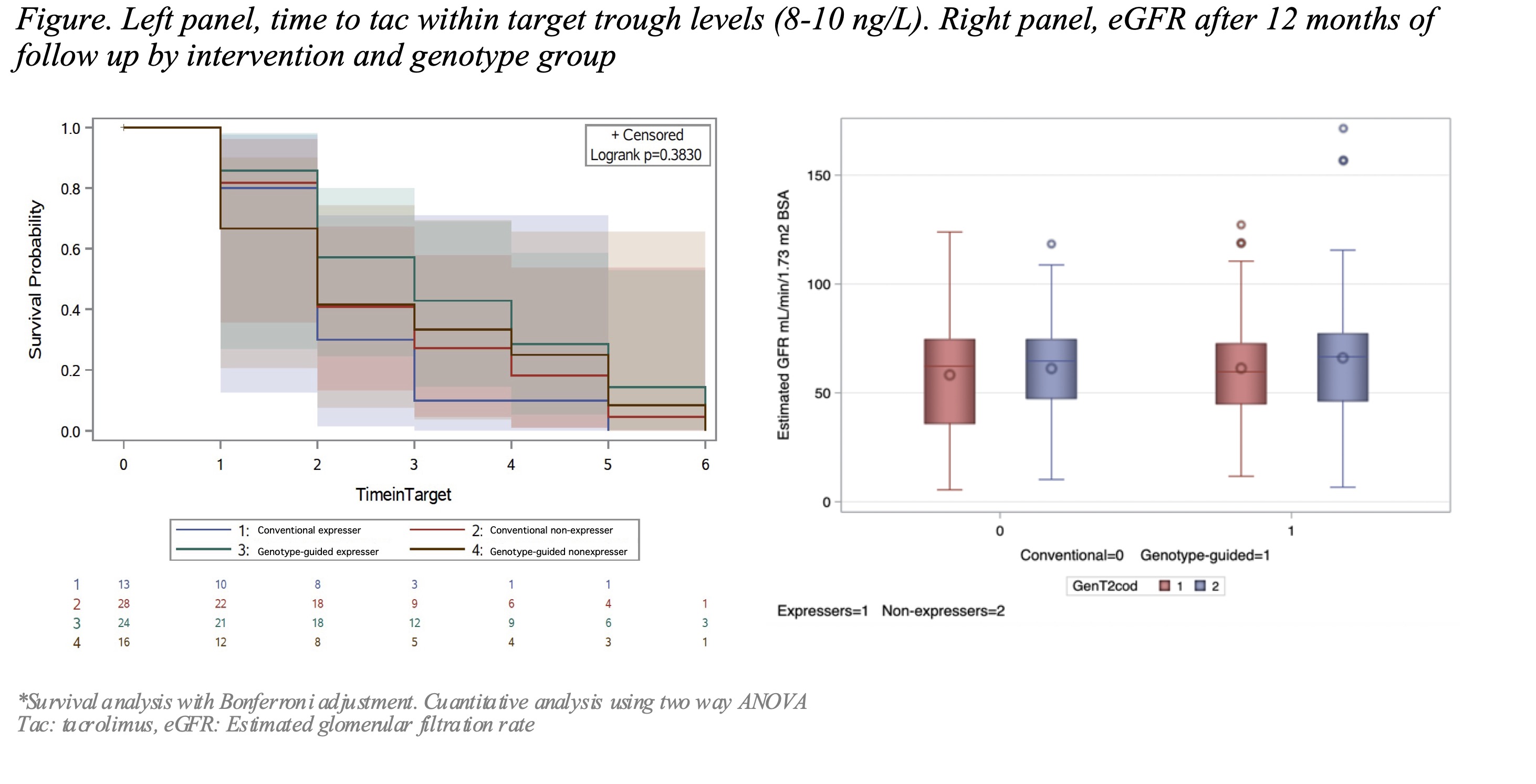
Results: We included 81 patients; 40 received the genotype-guided dose, the mean age at transplantation was 13.3 y/o, 51.8% were female. There was a higher frequency of expressers in the genotype-guided than the conventional-dose group (29.6 Vs 16.1% p=0.04), having a higher number of AA1*1. There were no differences in the time to achieve levels within-target (8-10 ng/L) logrank=0.182 between groups. There were no associations with rejection, OR 0.86 (95% CI 0.26-2.84, SE 0.606), or nephrotoxicity events, OR 5.85 (95% CI 0.60-56.8, SE 1.161) and the intervention. The eGFR remained similar (63.1 27.4 Vs 60.3 22.3 mL/min/1.73 m2 SC p=0.32) among groups. The subgroup analysis by genotype showed that expressers in the genotype-guided dose group took longer to achieve target levels (mean 5 vs 3.2 weeks, log-rank= 0.383).
Conclusions: Tacrolimus CYP3A5 genotype-guided initial dose after kidney transplantation did not show benefits on time to target levels, rejection, tac related nephrotoxic events or eGFR after a 12-months follow-up in our cohort. The higher frequency of expressers in the genotype-guided group could influence the results, showing a high impact of the polymorphisms. New dosing strategies are needed to improve accuracy.