Efficacy and safety of an intensified dosing regimen of enteric-coated mycophenolate sodium in de novo kidney transplant recipients in China: a prospective cohort study
Wenqing Xie1,2,3,4, Wenhan Peng1,2,3,4, Zhechi He1,2,3,4, Junhao Lv1,2,3,4, Wenhua Lei1,2,3,4, Rending Wang1,2,3,4, Hongfeng Huang1,2,3,4, Jianyong Wu1,2,3,4, Jianghua Chen1,2,3,4.
1Kidney Disease Center, the First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People's Republic of China; 2Kidney Disease Immunology Laboratory, State Administration of Traditional Chinese Medicine of China, Hangzhou, People's Republic of China; 3Institute of Nephrology, Zhejiang University, Hangzhou, People's Republic of China; 4Key Laboratory of Kidney Disease Prevention and Control Technology, Zhejiang Province, Hangzhou, People's Republic of China
Introduction: Previous study showed the standard dose of immunosuppressants enteric-coated mycophenolate sodium (EC-MPS) might result in unsatisfactory control rate of effective blood concentration and improvable acute rejection rate. This prospective cohort study was designed to evaluate the efficacy and safety of an intensified dosing regimen of EC-MPS in de novo kidney transplant recipients in China.
Methods: This prospective cohort study enrolled patients underwent de novo renal transplantation from 2015.6 to 2018.9. Participants were divided into the intensified-dose (week 1: 2160 mg/day; week 2: 1440 mg/day; followed by 720-1080 mg/day) and standard-dose (week 1: 1440 mg/day; week 2: 1080 mg/day; followed by 720-1080 mg/day) groups. Sub-group were analyzed based on mycophenolic acid (MPA)-area under the concentration (AUC): achieved group (defined as patients whose MPA AUC is equal or beyond 40 mg.h/L within Day 7) and under exposure group (defined as patients whose MPA AUC is below 40 mg.h/L within Day 7). The primary endpoints were 12-month-biopsy-proven acute rejection (BPAR), graft and patient survival rates. safety were also assessed. The study was followed up 3 years.
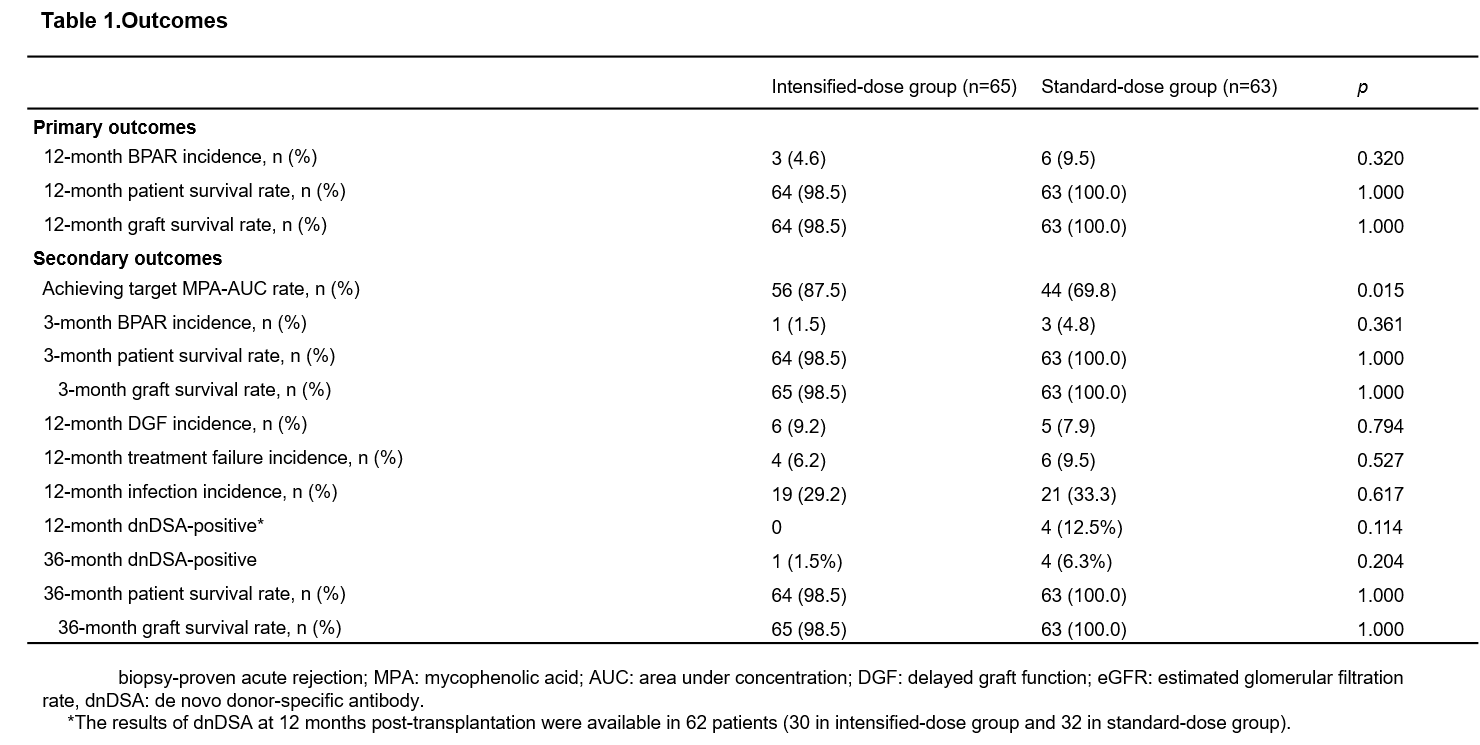
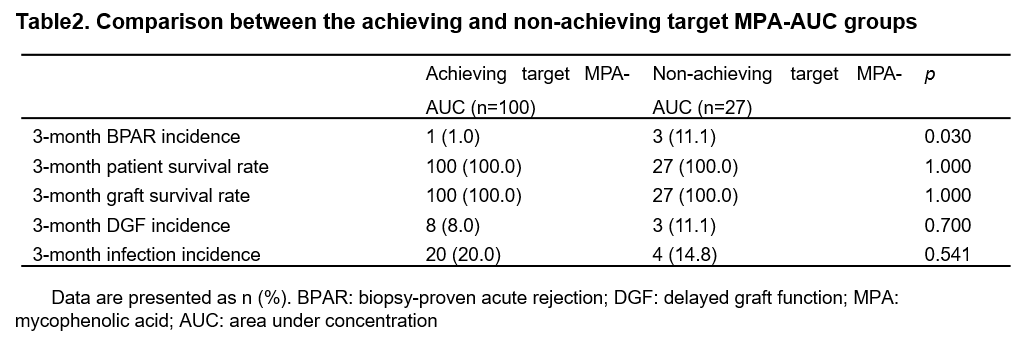
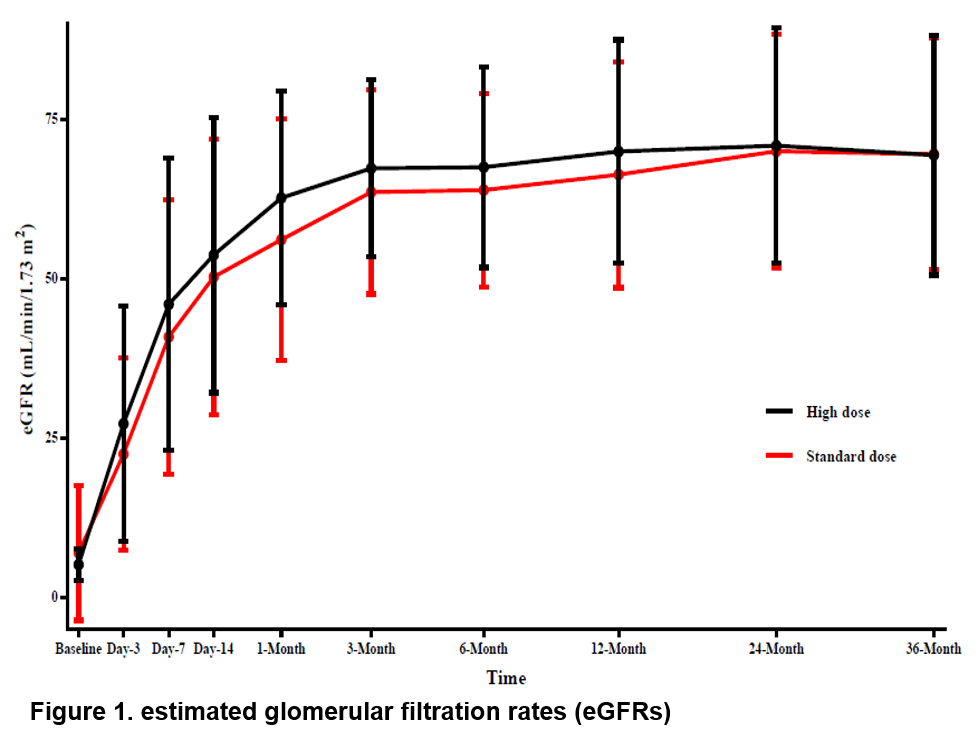
Results: A total of 128 patients were included, with 65 and 63 patients in the intensified-dose group and standard-dose group, respectively. The 12-month-BPAR incidence was numerically lower in the intensified-dose group than standard-dose group (4.6% vs. 9.5%, p=0.320). The rate of patients achieving target mycophenolic acid (MPA)-area under the concentration (AUC) was significantly higher in the intensified-dose group (87.5% vs. 69.8%, p=0.015) (Table 1) than standard-dose group, and patients achieving target MPA-AUC had a significantly lower 3-month-BPAR incidence (1.0% vs. 11.1%, p=0.03) (Table 2). patient and graft survival rates at 36 months between the intensified-dose and standard-dose groups was 98.5% vs. 100.0% and 98.5% vs. 100.0%, respectively. The 36-month- de novo donor-specific antibody (dnDSA) positive incidence rates was numerically lower in the intensified-dose group than standard-dose group (1.5% vs. 6.3%, p=0.204). In addition, delayed graft function, treatment failure, serum creatine levels, eGFRs and infection incidence had no significant differences between the two groups (all p>0.05) (Figure 1). The incidence rates of infection were similar in both groups (29.2% vs. 33.3%, p=0.617). No additional safety concerns related to intensified dosing of EC-MPS.
Conclusion: Intensified dosing of EC-MPS results in a numerically lower incidence of 12-month-BPAR and lower positive incidence of dnDSA with 36 months follow up. Intensified dosing could help achieve target therapeutic blood concentration of EC-MPS, that showed significantly lower BPAR and no more infection incidence increased and might further improve long-term prognosis in Chinese de novo kidney transplantation recipients.