Arthur A Cross-Najafi, United States has been granted the TTS International Transplantation Science Mentee-Mentor Awards
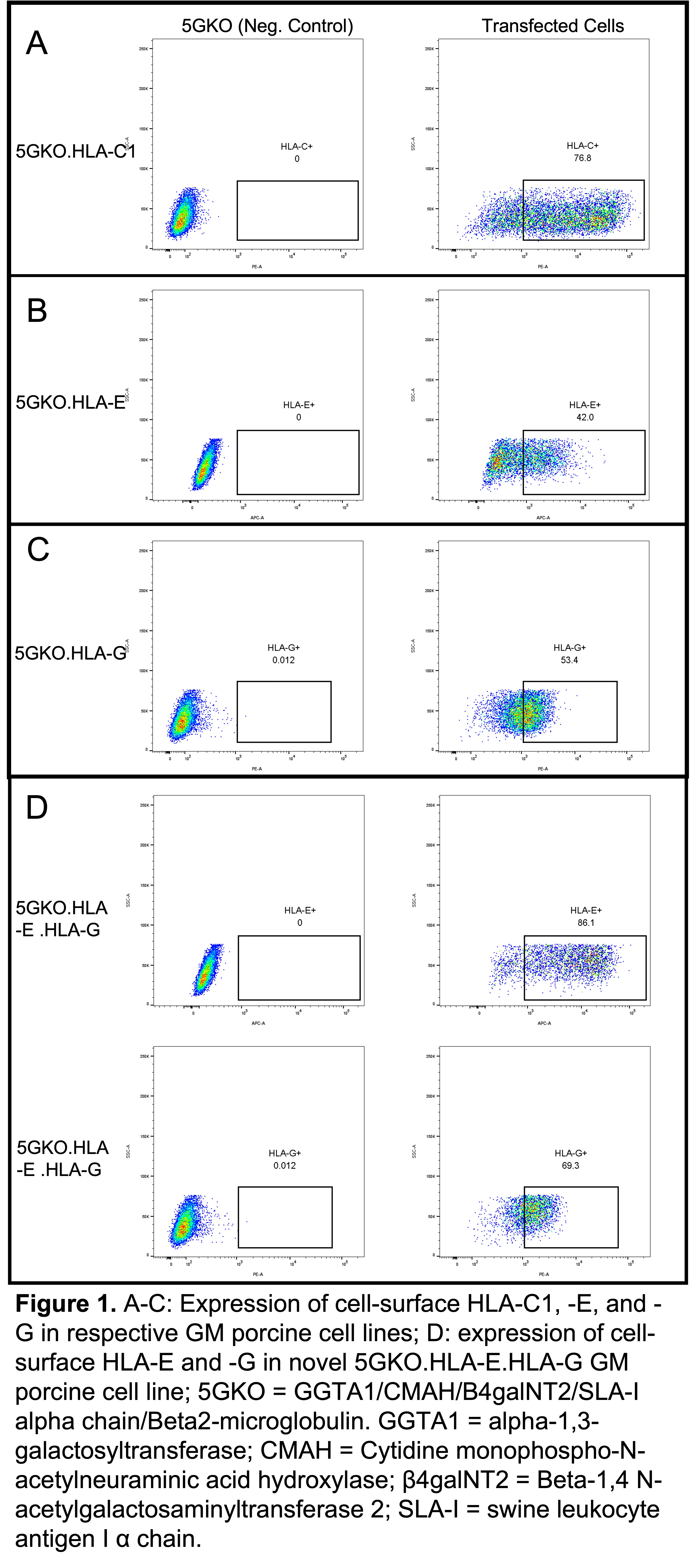
The role of co-expressing MHC class I molecules in pig endothelial cells: a case for HLA-E and HLA-G in xenotransplantation
Arthur Cross-Najafi1, Kevin Lopez1, Abdulkadir Isidan1, Yujin Park1, Wenjun Zhang1, Jonathan A Fridell1, Ping Li1, Burcin Ekser1.
1Division of Transplant Surgery, Department of Surgery, Indiana University School of Medicine, Indianapolis, IN, United States
Introduction: Pig-to-human xenotransplantation (XTx) is a promising solution to the organ shortage. Genetically-modified (GM) pigs lacking major xenoantigens have reduced hyperacute rejection and prolonged xenograft survival which further suggested XTx as a promising solution to the organ shortage. Despite these advancements, acute rejection remains a major barrier to clinical XTx. Natural killer cells (NK) play a crucial role in transplantation. In the context of XTx, suppressing human NK activity by expressing HLA class I molecules in pig organs may protect xenografts from host immune attack. Therefore, we aimed to (i) individually express HLA-C1, -E, and -G in a GM pig endothelial cell line null for five key xenoantigens (5GKO), (ii) co-express HLA-E and HLA-G in 5GKO, and (iii) evaluate human NK response to HLA class I+ 5GKO cells.
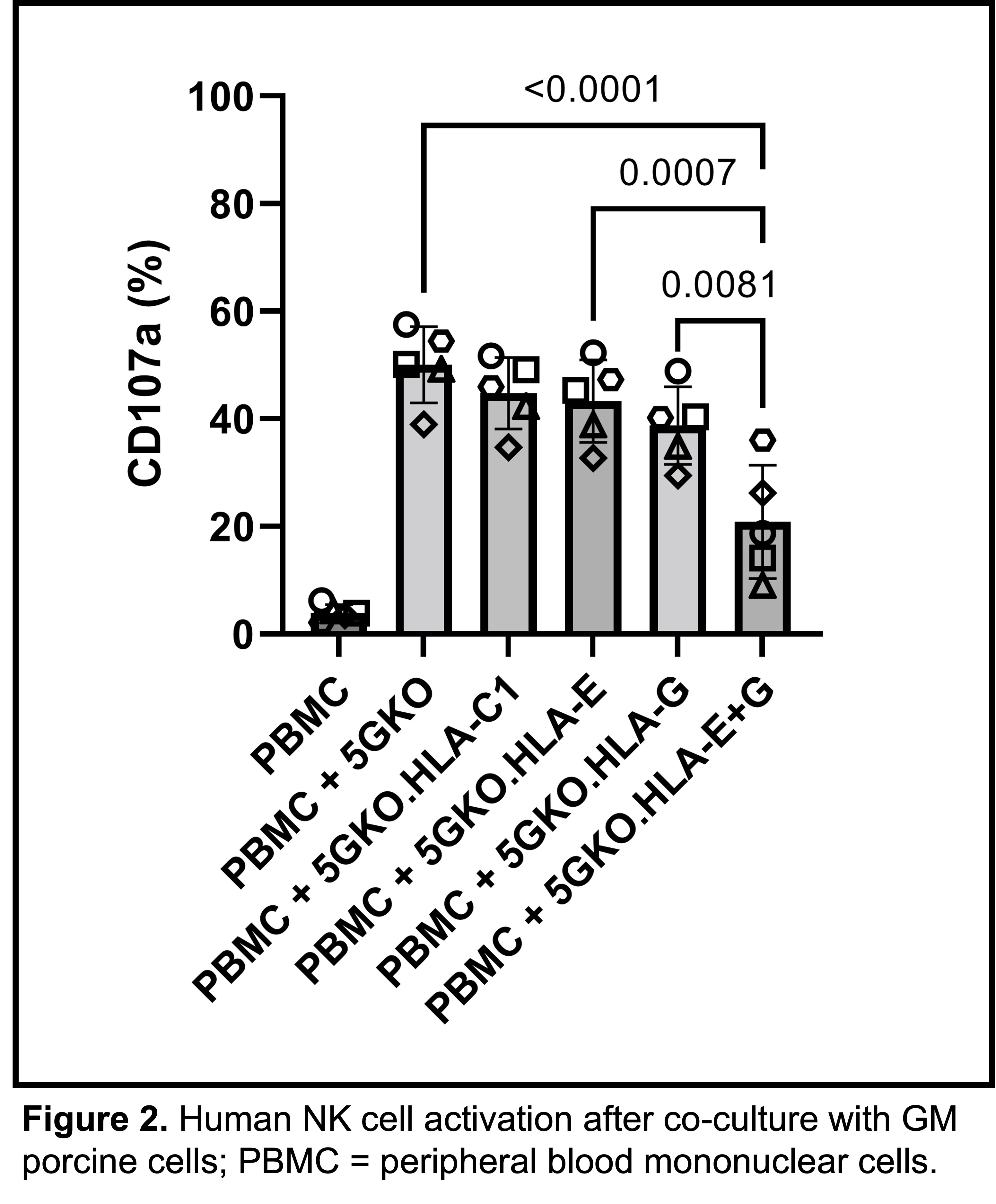
Methods: DNA sequences encoding HLA-C, -E, and -G were designed, synthesized, and cloned into expression vectors. Four GM porcine cell lines (5GKO.HLA-C1, 5GKO.HLA-E, 5GKO.HLA-G, 5GKO.HLA-E.HLA-G) were generated by transfection. IL-2-activated human peripheral blood mononuclear cells (PBMC) from five donors were co-cultured with GM porcine cell lines to initiate NK cell-mediated immune responses. Using flow cytometry, NK cell activation was evaluated by quantifying cell-surface expression of CD107a in the CD3-CD56+ cell population. Statistical analyses performed include one-way ANOVA and a post-hoc Tukey test.
Results: We successfully expressed HLA-C1, HLA-E, and HLA-G in 5GKO cells (Fig.1 A-C) and co-expressed HLA-E and HLA-G in 5GKO cells (Fig.1 D). Individual expression of HLA-C, -E, and -G did not demonstrate a significant difference in NK cell activation compared to control 5GKO cells; however, co-expression of HLA-E and HLA-G significantly reduced human NK cell activation compared to all other groups (Fig. 2).
Conclusion: Co-expression of HLA-E and HLA-G in porcine cells synergistically reduces NK cell-mediated cytotoxicity and shows promise for incorporation into GM pigs for pig-to-human xenotransplantation.
Work on xenotransplantation at Indiana University has been supported by internal funds of the Department of Surgery, in part, with support by the Board of Directors of the Indiana University Health Values Fund for Research Award (VFR-457-Ekser), the Indiana Clinical and Translational Sciences Institute, funded in part by Grant # UL1TR001108 from the National Institutes of Health (NIH), National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and NIH NIAID R21AI164002.
