Histological and molecular characterization of kidney xenografts transplanted to decedent humans
Alexandre Loupy3, Alessia Giarraputo3, Valentin Goutaudier 3, Blaise Robin3, Fariza Mezine3, Massimo Mangiola1, Jeffrey Stern1, Adam Griesemer1, Vasishta S Tatapudi1, Nicole Ali1, Sapna Mehta1, Zoe Stewart1, David Ayares2, Patrick Bruneval3, Robert Montgomery1.
1Transplantation, NYU Langone, New York, NY, United States; 2Revivicor, Inc, Blacksburg, VA, United States; 3Paris Transplant Group, INSERM, Paris, France
Background: Genetic modifications have recently permitted Xenotransplantation in decedent humans showing initial kidney function recovery. There is an unmet need and unprecedent opportunity for providing a precision phenotyping of these allografts to further optimize Xenotransplantation.
Methods: We report here the complete histological and immunological asessment of the first 2 Xenotransplants performed at NYU. Porcine renal Xenografts were procured from genetically engineered (α-Gal knockout) pigs. Kidneys were removed and wedged biopsied at 56 hours after transplantation and compared with non-transplanted genetically modified and WT pig kidneys. We performed a multiplex histological, histochemical consisting in staining for different cell types including CD20, CD3, CD15, CD 68, NKP46 and C4d. Expression analysis of RNA samples was performed using the nCounter platform. To capture both the host immune response coming from the receiver with the tissue-related pig genes, we adapted the B-HOT panel from cross-species analysis between Xenograft and Human by assigning the orthologous genes between corresponding human and pig data sets. The differential expression analysis was computed using Wald test and significative genes were identified after adjusting for multiple comparisons (Benjamini-Hochberg method).
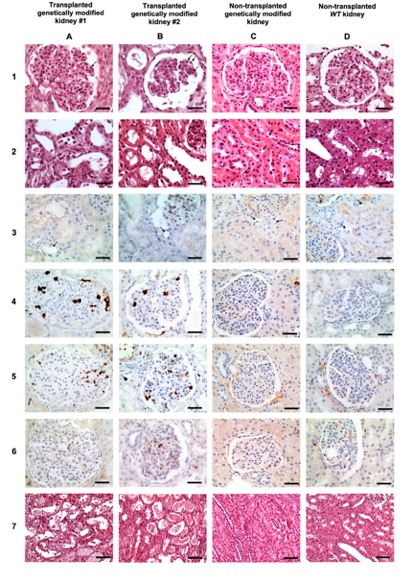
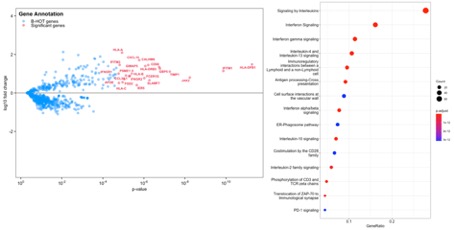
Results: Both Xenografts presented with glomerulitis lesions characterized by microvascular inflammation in glomerular capillaries composed by CD15+ and CD68+ macrophages cells. There were leukocytes infiltration of in peritubular capillaries of Xenograft #1 which involved < 10% of the cortex, while Xenograft#2 did not show ptc lesions. CD3+ T lymphocytes were very rare in glomeruli and interstitium and tubulitis was absent in both Xenografts. No C4d complement deposits was detected. Neither arteritis nor interstitial hemorrhage and thrombotic microangiopathy were observed (Figure 1). Both Xenografts showed acute tubular necrosis reflecting ischemia-reperfusion injury. Non transplanted kidneys from both Genetically modified pigs as well as wild type pigs did not show any abnormalities (Figure 1). Gene expression studies revealed in both Xenografts increased expression of biologically relevant genes related to injury repair response (APOE, TIMP1, IER5), endothelial activation (FGD2), effector T-cell (GIMAP5), IFNg response (CALHM6,GBP5,IFITM1,IFITM3, JAK2, PSME1, SLAMF7, CXCL10), monocyte/macrophage activation (CD68, IFNGR1, IFNGR2, FCER1G), and humoral response (HLA-A, HLA-B, HLA-C, HLA-DPB1, HLA-DRB3, CCL3/L1), Figure 2.
Conclusions: This first multiplex xenograft assessment reveals adequate Xeno allografts without hyperacute rejection. However, we found a pattern of ongoing antibody-mediated rejection confirmed by gene expression showing increased expressing of injury genes as well as endothelial, macrophages and AMR genes. These results open avenues for future refinement and targets for improving xenograft both from allograft preservation to the control of the humoral harm of rejection.