Investigating galectin-3 as a novel therapeutic target to block inflammation and reduce ischemia reperfusion injury during vascular composite allograft transplantation
Zhaohui Wang1, Dor Yoeli1, Niyati Nakra1, Joy Huang1, Bing Li1, Yong Wang1, Nathaly Limon de la Rosa1, An-Jey Su1, Alkesh Jani1, David W Mathes1, Kia Washington1, Evan Farkash2, Christene A Huang1.
1Surgery, University of Colorado Anschutz Medical Campus, Aurora, CO, United States; 2Pathology, Michigan Medicine, University of Michigan, Ann Arbor, MI, United States
Introduction: Vascularized Composite Allograft (VCA) transplantation is a treatment option for complex injuries that leave patients with structural and functional deficits that cannot be adequately reconstructed. During transplantation, grafts are subjected to hypoxic/ischemic injury during procurement, storage, and reperfusion. Skin and muscle containing VCA are highly susceptible to ischemia reperfusion injury. Galectin-3 is an endogenous β-galactoside binding lectin known to play a role in driving inflammatory responses in response to hypoxic/ischemic stress. Blocking galectin-3 using known inhibitors including modified citrus pectin (MCP) has been shown in animal models to reduce inflammation and fibrosis. We hypothesized that galectin-3 significantly contributes to VCA IRI and that blocking galectin-3 can serve as a novel therapeutic approach to prevent or reduce IRI. We aimed to determine the role of galectin-3 in VCA ischemia reperfusion injury and the effect of blocking galectin-3 using MCP in syngeneic VCA models.
Method: Male Brown Norway rats underwent syngeneic hind limb transplantation following 0, 6 or 24 hours of cold ischemia time. A group of rats receiving hind limbs subjected to 24 hours of static cold storage were treated with MCP (1% w/v) in the drinking water starting one week prior to transplantation. Recipient serum and donor VCA tissues were collected at end-of-study 6 days post transplantation. Sera and tissues collected from naive Brown Norway rats were included as controls. Sera levels of galectin-3 were determined by sandwich ELISA (Novus Biologics, Inc). Galectin-3 expression in the muscle was determined by Western Blotting. The degree of inflammation in the muscle tissue was determined by H&E.
Results: Sera galectin-3 levels averaged 0.854±0.557ng/ml in naïve BN rats (n=7), 4.232±2.134ng/ml in recipients of grafts transplanted immediately without static cold storage (n=3), 12.86±1.292ng/ml in recipients of grafts subjected to 6 hours cold storage without MCP treatment (n=3),20.10±3.185ng/ml in recipients of grafts subjected to 24 hours cold storage without MCP treatment (n=6), and 12.77±2.098ng/ml in recipients of grafts subjected to 24 hours cold storage with MCP treatment (n=9) (Figure 1A). Galectin-3 expression in the VCA muscle increased according to the extent of cold ischemia. MCP treatment decreased muscle galectin-3 expression (Figure 1B) and inflammation in the muscle (Figure 2) post transplantation.
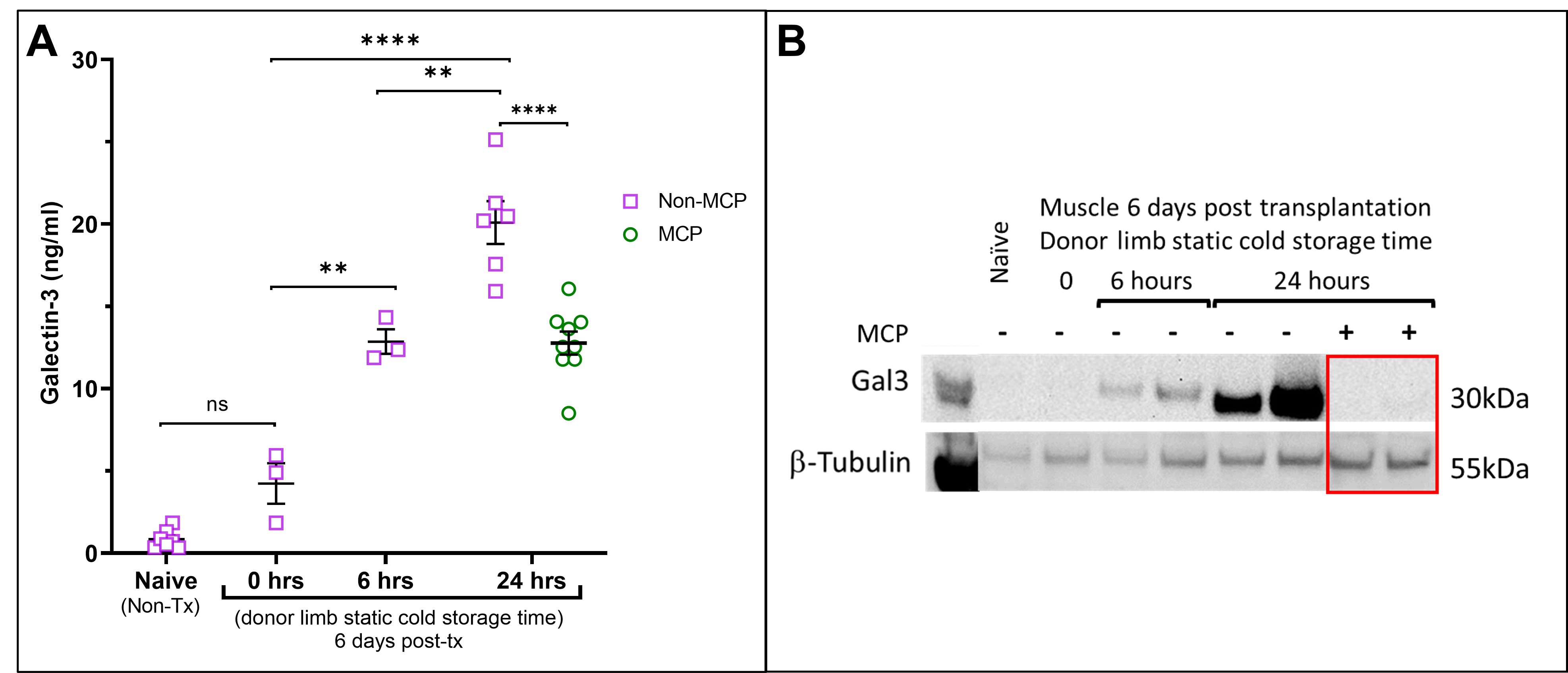
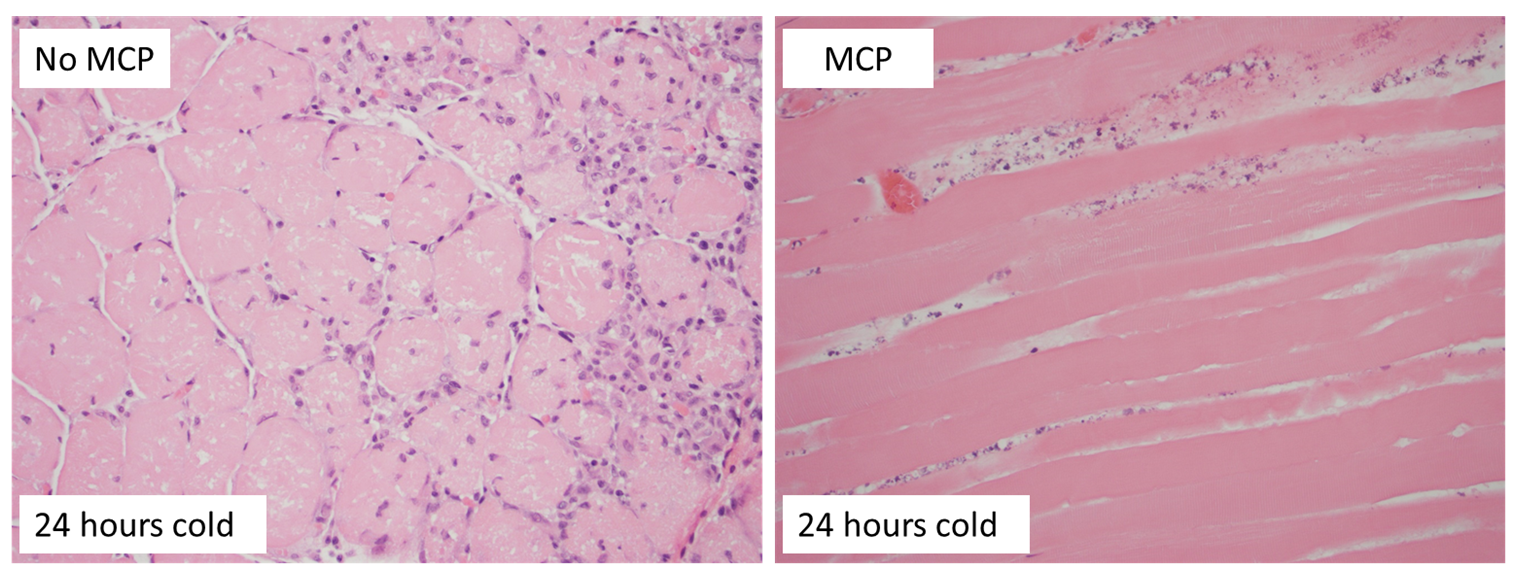
Conclusion: Galectin-3 levels significantly increased in recipient sera and donor muscle within a week in rats transplanted with donor VCA grafts subjected to prolonged ischemia. Blocking galectin-3 using MCP can reduce the amount of galectin-3 in circulation and muscle tissue post transplantation and may decrease inflammation associated with VCA IRI. Galectin-3 may serve as a novel therapeutic target to reduce inflammation associated with ischemic injury and improve VCA outcomes.
DOD CDMRP RTRP W81XWH1910163 RT180168.
