Beneficial addition of donor-derived cell-free DNA testing in pediatric kidney transplantation
Jayanthi Chandar1, Marissa Defreitas1, Renata Glehn-Ponsirenas2, George Burke III3.
1Pediatrics, Division of Pediatric Nephrology, University of Miami, Miller School of Medicine, Miami Transplant Institute, Miami, FL, United States; 2Medical Affairs, CareDx, San Francisco, CA, United States; 3Surgery, Division of Transplant Surgery, University of Miami, Miller School of Medicine, Miami Transplant Institute, Miami, FL, United States
Background: Donor-derived cell- free DNA (dd-cfDNA) is a useful plasma biomarker to assess allograft injury and rejection in kidney transplant recipients (KTRs). Serial monitoring of dd-cfDNA is recommended to monitor allograft rejection and to determine adequacy of immunosuppression. Although, serum creatinine increase is a marker for allograft dysfunction, it is limited by variations in hydration status, increased muscle mass, and growth in children. Herein, we describe a single-center experience on 1) the indications for performing dd-cfDNA fraction in pediatric KTRs and 2) comparison of dd-cfDNA fraction in various clinical states.
Methods: This is a cross-sectional observational single-center study in pediatric KTRs who were tested for dd-cfDNA in plasma for a clinical indication. Children were categorized according to the indication for testing and the median values of dd-cfDNA were noted for each category. Comparisons were made by Kruskal Wallis test between each category. P <0.05 was considered significant. Receiver Operating Characteristic (ROC) curve analysis was performed to analyze the sensitivity and specificity of dd-cfDNA to predict biopsy-proven graft injury.
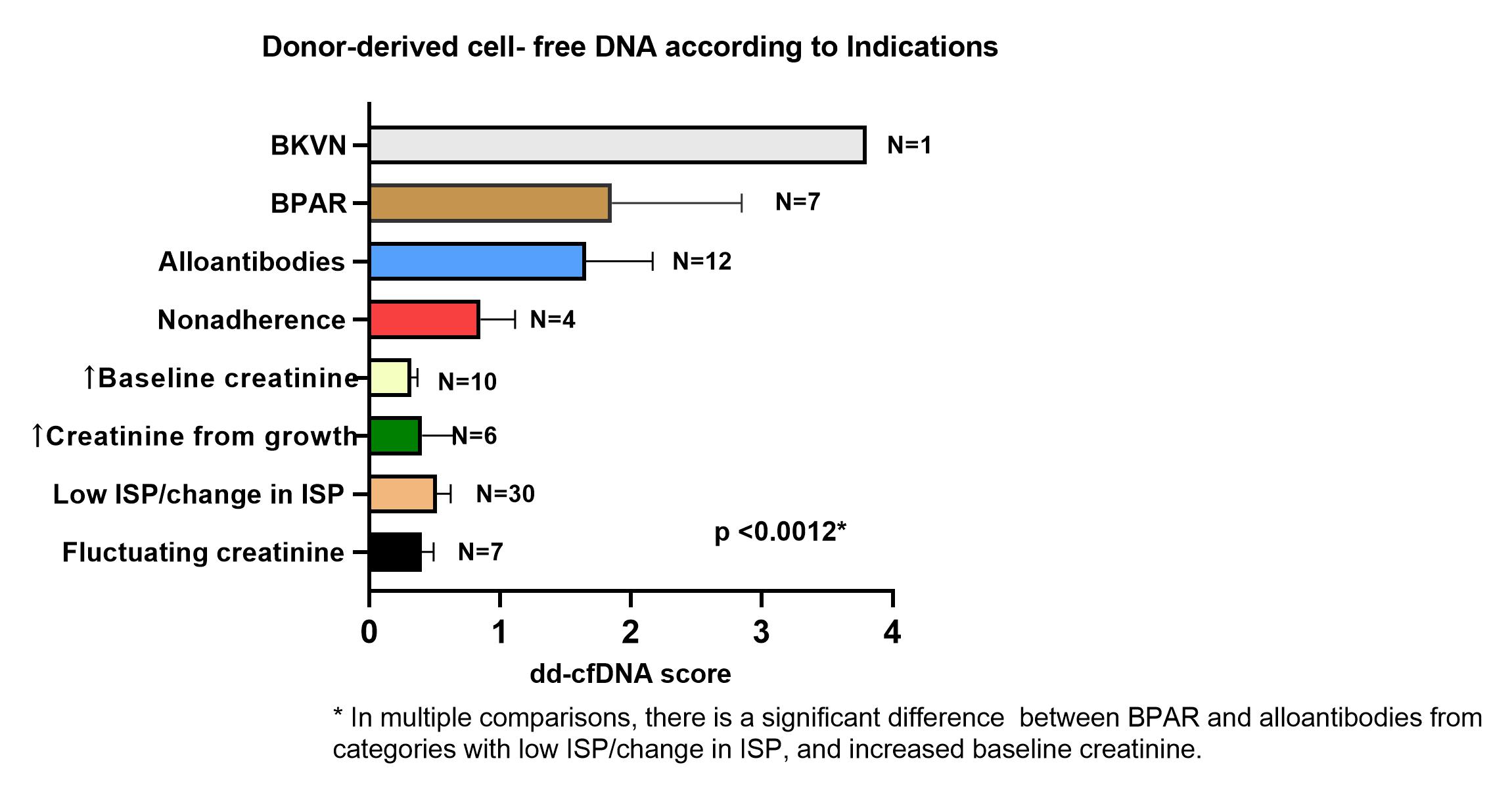
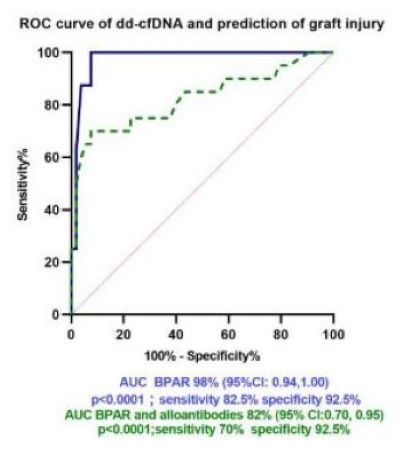
Results: dd-cfDNA was performed in 77 children transplanted at the Miami Transplant Institute with a mean age of 11.6 ± 5.9 years; 54 (70%) were male. Seven (9%) were second transplants. Mean time from transplant to time of dd-cfDNA testing was 3.5 ± 3.2 years (range 1 month to 19 years). Indications for testing included highly sensitized state or development of alloantibodies (N=12), clinical suspicion of rejection (N=7), BK nephropathy (N=1), low immunosuppression because of leukopenia, viral replication or recurrent bacterial infections (N=30), fluctuating serum creatinine (N=7), increased serum creatinine with normal growth N=6), increased post-transplant baseline creatinine for age (N=10) and non-adherence (as evidenced by low tacrolimus levels without change in kidney function; N=4). There was a significant difference in dd-cfDNA between children who had biopsy-proven acute rejection (BPAR) rejection, BK virus nephropathy (BKVN) and those who had alloantibodies (median 1.35 (95% CI 0.6,2.4) versus those who did not [median 0.3 (95%CI 0.25, 0.43); p =0.0012]. Children who were on lower levels of immunosuppression because of leukopenia, viral or bacterial infections had low dd-cfDNA [median score: 0.32 (95%CI 0.25, 0.45); Figure 1]. ROC curve analysis revealed high specificity and sensitivity for dd-cfDNA to predict graft injury and presence of alloantibodies (Figure 2).
Conclusions: dd-cfDNA is a clinically useful adjuvant tool to determine allograft injury in pediatric kidney transplant recipients. Traditionally, immunosuppressive drug levels have been used to determine immunosuppression dosing. dd-cfDNA helps distinguish immune activation versus immune quiescence and has the potential to guide and serially monitor immunosuppression dosing.