Assessment of donor derived cell free DNA (ddcfDNA) at surveillance and at clinical suspicion of acute rejection in renal transplantation
Evangelos Mantios1, Vassilis Filiopoulos1, Pantelis Konstantoulakis2, George Liapis3, Aggeliki Vittoraki4, Smaragdi Marinaki1, Ioannis Boletis1.
1Department of Nephrology and Kidney Transplantation, National and Kapodistrian University of Athens, General Hospital of Athens Laiko, Athens, Greece; 2Molecular Genetic and Cytogenetics, ”Genotypos” Science Laboratory, Athens, Greece; 3Pathology Department, National and Kapodistrian University of Athens, General Hospital of Athens Laiko, Athens, Greece; 4Immunology Department, National Tissue Typing Center, General Hospital of Athens "G.Gennimatas", Athens, Greece
Introduction: In order to study the efficacy of less pervasive methods to screen patients after renal transplantation, we tried to assess the correlation between ddcfDNA and acute rejection, cellular (TCMR) or antibody mediated rejection (ABMR) in recipients of renal allograft.
Method: Two groups of renal transplanted patient were assessed. The first group contained newly transplanted patients, from whom samples were collected at month 1, 2, 3 & 5 for ddcfDNA analysis, along with creatinine/eGFR and DSA monitoring. The second group was consisted of patients who underwent a renal biopsy for any cause and whose ddcfDNA were measured at the time of biopsy and 4 weeks afterwards. The biopsy group was further divided in two subgroups, patients diagnosed with rejection (ABMR and TCMR) and patients without rejection. Levels of ddcfDNA were also compared to serum creatinine and eGFR levels. The follow up of the patients lasted for one year. Measurements of dd-cfDNA were done using AlloSeq cfDNA kit (CareDx).
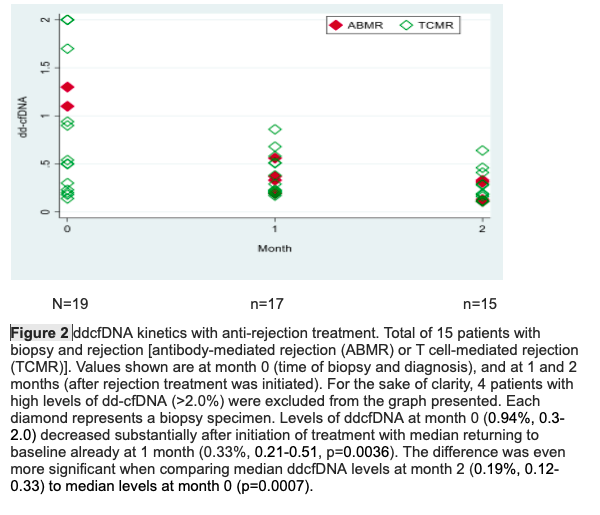
Results: The first group contained 30 patients (13 males), mean age 46.5±10.8 years, 6 patients who underwent ΑΒΟ incompatible transplantation and 7 with preformed DSA. The second group was consisted of 32 patients (21 males), mean age 41.5±14.3 years, 7 patients who underwent ΑΒΟ incompatible transplantation and 14 with preformed DSA. There was a statistical significance in ddcfDNA between patients who were diagnosed with acute rejection (median value 0.94%, IQR 0.3-2) and those without rejection (0.24% IQR 0.2-0.34) (p=0.004), while there was no significant difference in the serum creatinine. When a ddcfDNA threshold of 0.5% was chosen, it had a sensitivity of 73.7% and a specificity of 92.3% for the diagnosis of rejection (AUC: 0.80, 0.65-0.96) (Fig.1). Significant difference in ddcfDNA was also noticed among patients with ABMR (median value 13%, IQR 1.3-16), those with TCMR (0.52%, 0.23-1.70) and those without rejection (0.24%, 0,20-0,34) (p=0.0014). No difference was found in ddcfDNA among patients with borderline rejection, TCMR1 or TCMR2. When looking at newly transplanted patients with persistently elevated ddcfDNA (AlloSeq >1 result, ≥0.5%), the ddcfDNA level cut-off of 0.5% was not able to predict any difference in eGFR from month 5 to month 12. Finally, levels of ddcfDNA in the rejection subgroup before biopsy (0.94%, 0.3-2.0) decreased significantly after initiation of treatment with median returning to baseline already at 1 month (0.33%, 0.21-0.51, p=0.0036) (Fig.2).
Conclusion: Our study shows that ddcfDNA is a reliable biomarker for the diagnosis and monitoring of treatment of acute rejection, especially of antibody mediated rejection, as well as a useful screening tool for the patients who will require a renal allograft biopsy in the future.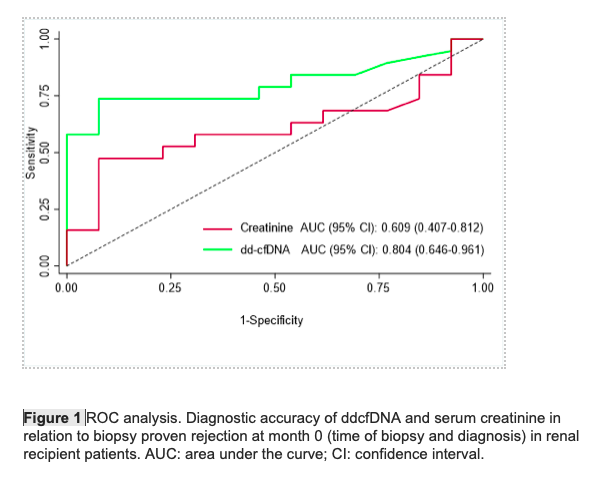
CareDx.
