
Achieving localized immunosuppression through ex vivo engineering of organ blood vessels
Daniel Luo1,2, Erika MJ Siren1,2, Winnie Enns3, Lyann Sim1, Franklin Tam3, Javairia Rahim3, Caigan Du4, Dicken Ko5, Steve Withers1, Jonathan C Choy3, Jayachandran N Kizhakkedathu1,2,6.
1Chemistry, University of British Columbia, Vancouver, BC, Canada; 2Centre for Blood Research & Life Sciences Institute, The University of British Columbia, Vancouver, BC, Canada; 3Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, BC, Canada; 4Urologic Sciences, The University of British Columbia, Vancouver, BC, Canada; 5Surgery, Warren Alpert Medical School of Brown University, Providence, RI, United States; 6Biomedical Engineering, The University of British Columbia, Vancouver, BC, Canada
Introduction: Classic immunosuppressants lead to systemic immune shutdown, though necessary to mediate transplant rejection, it may lead to various complications. To reduce off-target immunosuppression while retaining increased organ survival, we propose direct modification of the endothelium of vascular transplants ex vivo to achieve localized immunomodulation. The glycocalyx (eGcx), made up of membrane-bound glycoproteins, is of particular interest due to its ability to control cell to cell communication and the activation of immune response through cell recognition. During organ transplantation, inflammation and oxidative damage occurs and can lead to the shedding and damage of the eGcx layer; this has been linked to organ failure and rejection. We developed an enzymatic approach to modify the surface of the endothelium with immunosuppressive polymers to induce immunomodulatory effects locally. We tested the efficacy of this approach in murine transplants.
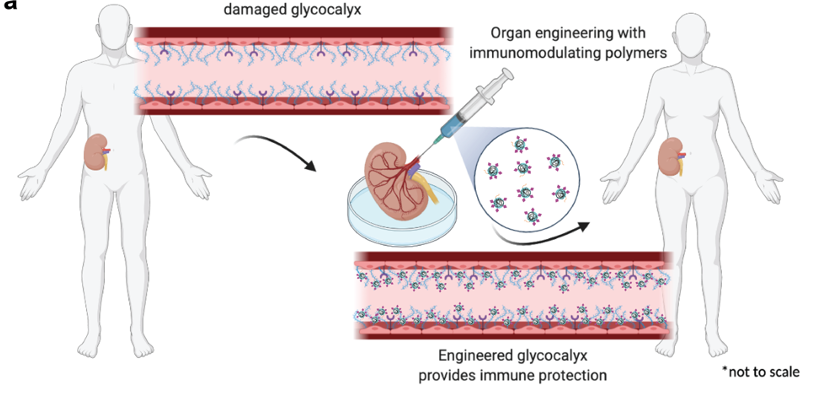
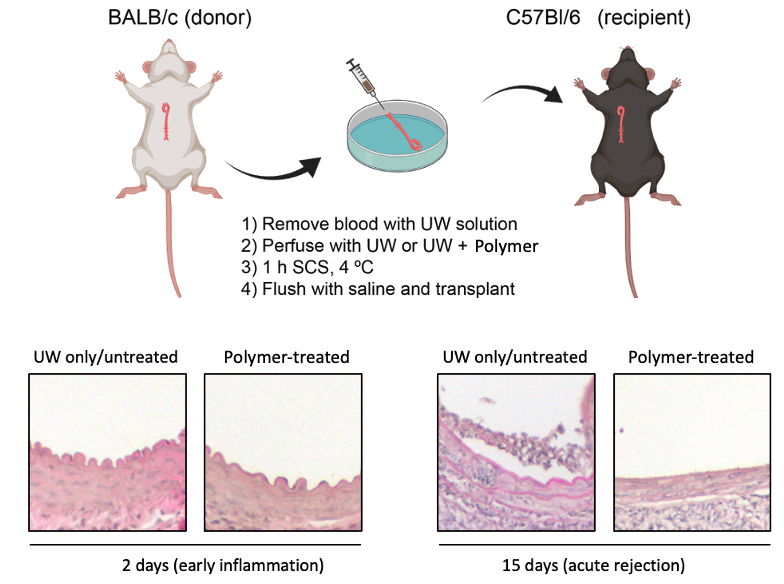
Method: We developed a method using tissue transglutaminase (tTGase) as the surface immobilizing enzyme and polyglycerol polymers containing sialic acid or sulfate moieties that is compatible with UW organ preservation solution at 4°C. In vitro mechanistic studies were performed using EaHy.926 cells to replicate the endothelium and PBMCs and CAR-T cells were used to test immune cytotoxicity. In vivo efficacy of graft rejection was assessed through aortic vessel or renal grafts from BALB/c donor mice into C57BL/6 recipient mice. Polymer treated and untreated grafts were assessed by serology and histology at various timepoints (day 2, 15 and 42 for vessel grafts and day 30 for renal grafts).
Results: In vitro, polymer modified endothelial cells were able to evade CAR-T cell induced cytoxicity and reduced oxidative stress. Moreover, polymer treatment reduced TNF release in M1 macrophages. In vivo, modified grafts showed reduced medial thickening and leukocyte infiltration in vessel transplants; further confirmed in the reduction of pro-inflammatory cytokines in serum. In 42 day studies, donor-specific antibody was reduced in polymer-treated grafts compared to untreated. Finally, histological analysis of polymer-treated renal grafts revealed less infiltration and mesangial expansion relating to a healthier graft after 30 days.
Conclusion: Here, the use of a polymer-mediated organ engineering approach leads to vascular protection that prevents immune-mediated rejection of organ transplants. Ex-vivo delivery of these immune cloaking polymers that engineer the blood vessel lumen allow for localized immune protection, making this an enticing and viable strategy for the reduction in the use of broad-active immunosuppressants post-transplantation. The protocol remains simple and easy to deliver, thereby enhancing its potential clinical applicability. To further validate this novel approach, studies in larger animal models that more closely replicate human transplant conditions are being planned.
Canadian Institutes of Health Research. Natural Sciences and Engineering Council of Canada. Heart and Stroke Foundation of Canada. Canadian Glycomics Network, GlycoNet.
