Single-cell immune profiling of human intestinal allografts reveals differential phenotypes of alloreactive T-cell clones in quiescent vs chronically rejecting allografts
Jianing Fu1, Zicheng Wang2, Mercedes Martinez3, Wenyu Jiao1, Kristjana Frangaj1, Rebecca Jones1, Xinzheng V. Guo4, Ya Zhang4, Wan-I Kuo4, Aleksandar Obradovic1, Wenji Ma2, Kortney Rogers1, Tomoaki Kato5, Yufeng Shen2, Megan Sykes1,5,6.
1Columbia Center for Translational Immunology, Department of Medicine, Columbia University, New York, NY, United States; 2Center for Computational Biology and Bioinformatics, Department of Systems Biology, Columbia University, New York, NY, United States; 3Department of Pediatrics, Columbia University, New York, NY, United States; 4Human Immune Monitoring Core, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, United States; 5Department of Surgery, Columbia University, New York, NY, United States; 6Department of Microbiology & Immunology, Columbia University, New York, NY, United States
Introduction: The success of intestinal transplantation (ITx) is limited by high rejection rates. We have demonstrated that host-vs-graft (HvG) alloreactive T-cell clones are enriched in intestinal allografts during early rejection and persist despite rejection resolution. Recipient T cells in the mucosa eventually take on resident memory T cell (TRM) features, possibly posing a constant threat of late rejection.
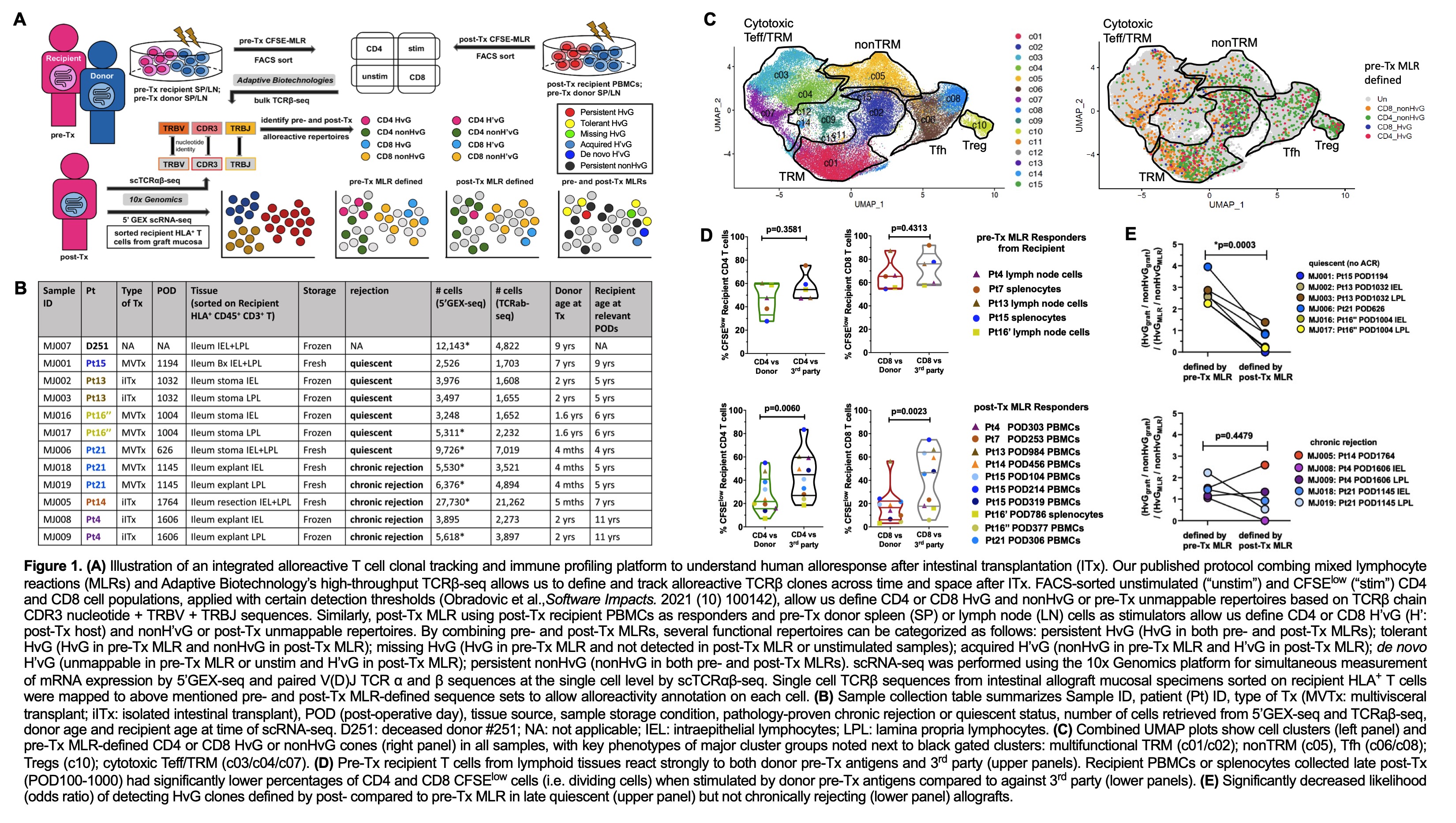
Methods: We integrated clonotype, alloreactivity and gene expression profiles of FACS-sorted allograft recipient T cells to assess the functional phenotype of donor-reactive mucosal T cells in association with graft outcomes using the 10x single cell RNA sequencing platform and our method for identifying donor-reactive TCRs from pre- and post-Tx mixed lymphocyte reactions (MLRs) (Fig.1A).
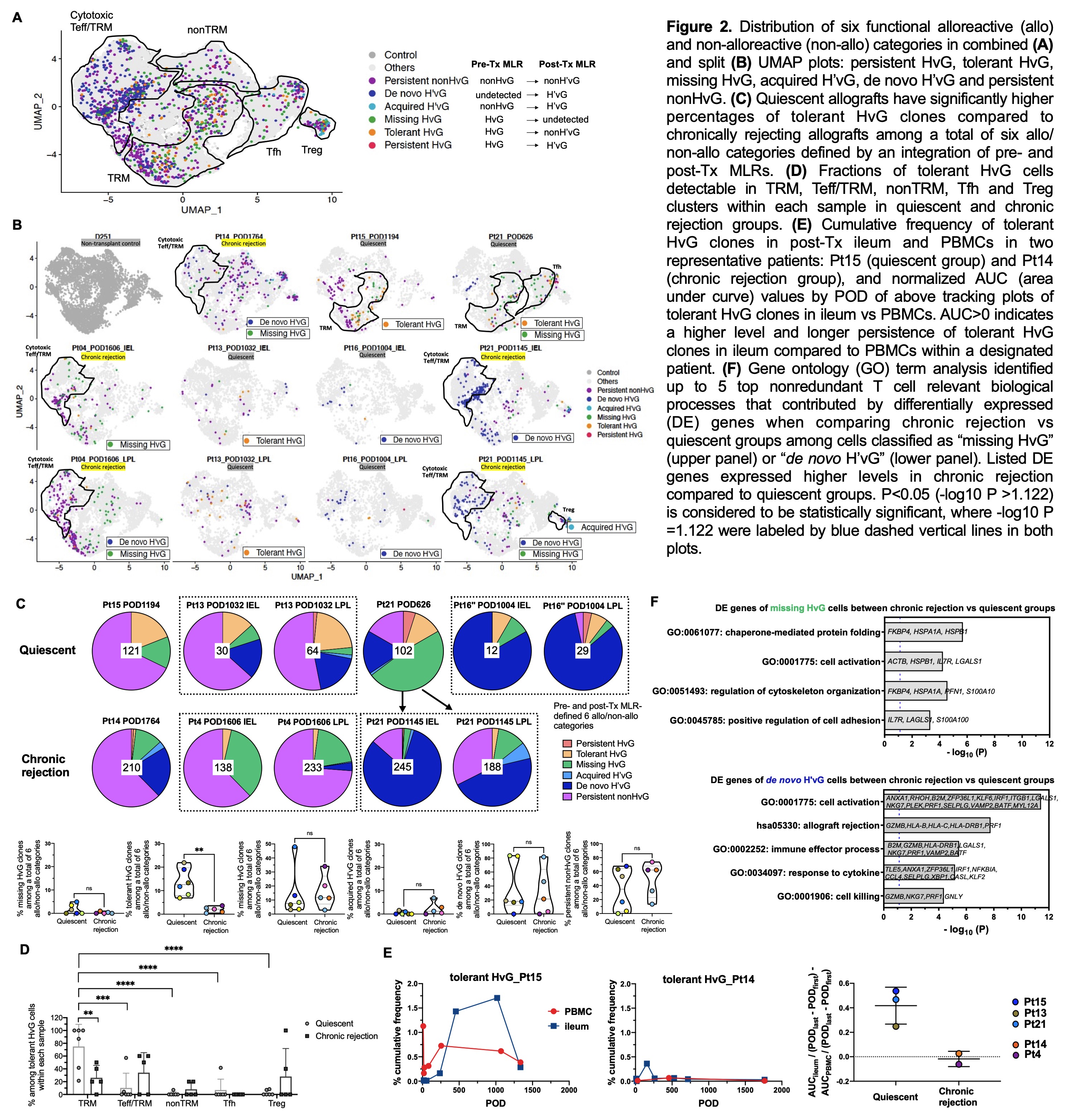
Results: Recipient mucosal T cells from 6 quiescent and 5 chronically rejecting allograft specimens and a non-transplant control intestine shared at least five transcriptionally-defined clusters (Fig.1B-C): multifunctional (IL17A+, IL22+, TNF+) TRMs (CD69+, ITGAE+, CXCR6+); cytotoxic γδ and CD8 αβ T cells with mixed effector T cell (Teff) and TRM features (CD69dim, RUNX3+, TBX21+, GZMB+); follicular helper T cells (Tfh: CXCR5+, PDCD1+, Bcl6+); nonTRMs (CCR7+, KLF2+, S1PR1+); and regulatory T cells (FOXP3+, CTLA4+). Pre-Tx MLR-defined HvG clones were mainly detected in TRM, Teff/TRM and Tfh clusters in both quiescent and chronically rejecting allografts. Hyporesponsiveness of circulating recipient T cells to donor vs third-party antigens in post-Tx MLR indicated partial tolerance of circulating recipient T cells to donor antigens post-Tx (Fig.1D). There was a significantly decreased likelihood of detecting HvG clones defined by post- compared to pre-Tx MLR in late quiescent (but not chronically rejecting) allografts (Fig.1E). Quiescent allografts contained a significantly greater percentage of pre-Tx HvG clones that became tolerant in post-Tx MLR (tolerant HvG) compared to chronically rejecting allografts (Fig.2A-C). Tolerant HvG among quiescent allografts were dominated by TRM profiles. The fraction of tolerant HvG cells in TRM clusters was significantly higher in quiescent compared to rejecting allografts (Fig. 2D). Patients with quiescent allografts tended to show a higher level and longer persistence of tolerant HvG clones in ileum compared to PBMCs (Fig.2E). Cells classified as missing HvG (HvG in pre-Tx MLR and not detected in post-Tx MLR or circulation) and de novo H’vG (unmappable in pre-Tx MLR and unstimulated cells but HvG in post-Tx MLR) in chronically rejecting allografts showed significantly higher expression of genes related to cytotoxic Teff functions compared to those in quiescent allografts (Fig.2F).
Conclusion: Single cell immune profiling reveals distinct contributions of pre-existing HvG-reactive T cells that potentially became tolerant in allografts with TRM transcriptional profiles in quiescent vs chronically rejecting allografts.