Elevated donor-derived cell-free DNA (dd-cfDNA) in the early post-transplant period is associated with an increased incidence of adverse clinical outcomes in kidney transplant recipients
David Wojciechowski2, Anup Patel4, Sanjiv Anand3, Jeffrey Klein6, Anil Paramesh8, Puneet Sood7, Nikhil Agrawal1, Grigory Shekhtman1, Mingwei Fei1, Kunbin Qu1, Daniel Brennan5.
1Kidney Transplant, CareDx, Brisbane, CA, United States; 2Medicine, UT Southwestern, Dallas, TX, United States; 3Medicine, Intermountain Healthcare, South Salt lake, UT, United States; 4Medicine, Saint Barnabas Medical Center, Livingston, NJ, United States; 5Medicine, Johns Hopkins University, Baltimore, MD, United States; 6Medicine, University of Kansas, Kansas City, KS, United States; 7Medicine, University of Pittsburgh, Pittsburgh, PA, United States; 8Surgery, Tulane University, New Orleans, LA, United States
KOAR registry.
Introduction: Early post-transplant elevations in dd-cfDNA, even in the absence of histologic rejection or other overt pathology, have been suggested to carry a riskof adverse outcomes among solid organ transplant recipients. We investigated this association among kidney transplant recipients enrolled in the Kidneyallograft Outcomes AlloSure Registry (KOAR, NCT03326076).
Methods: To assess the impact of early post-transplant dd-cfDNA elevations, we evaluated the incidence of a clinical composite that included biopsy-provenrejection (BPAR), detection of de novo donor specific antibodies (dnDSA) and return to dialysis in patients with and without median dd-cfDNA >1% over the first100 days post-transplant. Patients with events before day 100 were excluded. Univariate and multivariate analyses were performed.
Results: 51 of 1296 patients (3.9%) had a median dd-cfDNA ≥1.0% during the first 100 days post-transplant. In a univariate model, these patients had asignificantly higher risk of the experiencing both the composite outcome and each individual component during the first post-transplant year (Figure 1). In a multivariate Cox proportional hazards model that included recipient age, delayed graft function, donor type (living vs deceased), and recipient sensitization,only 100-day median dd-cfDNA elevation ≥1.0% was a statistically significant predictor of the composite outcome, with a hazard ratio of 2.99 (95% CI: 1.59 -5.61, p < 0.005) (Table 1).
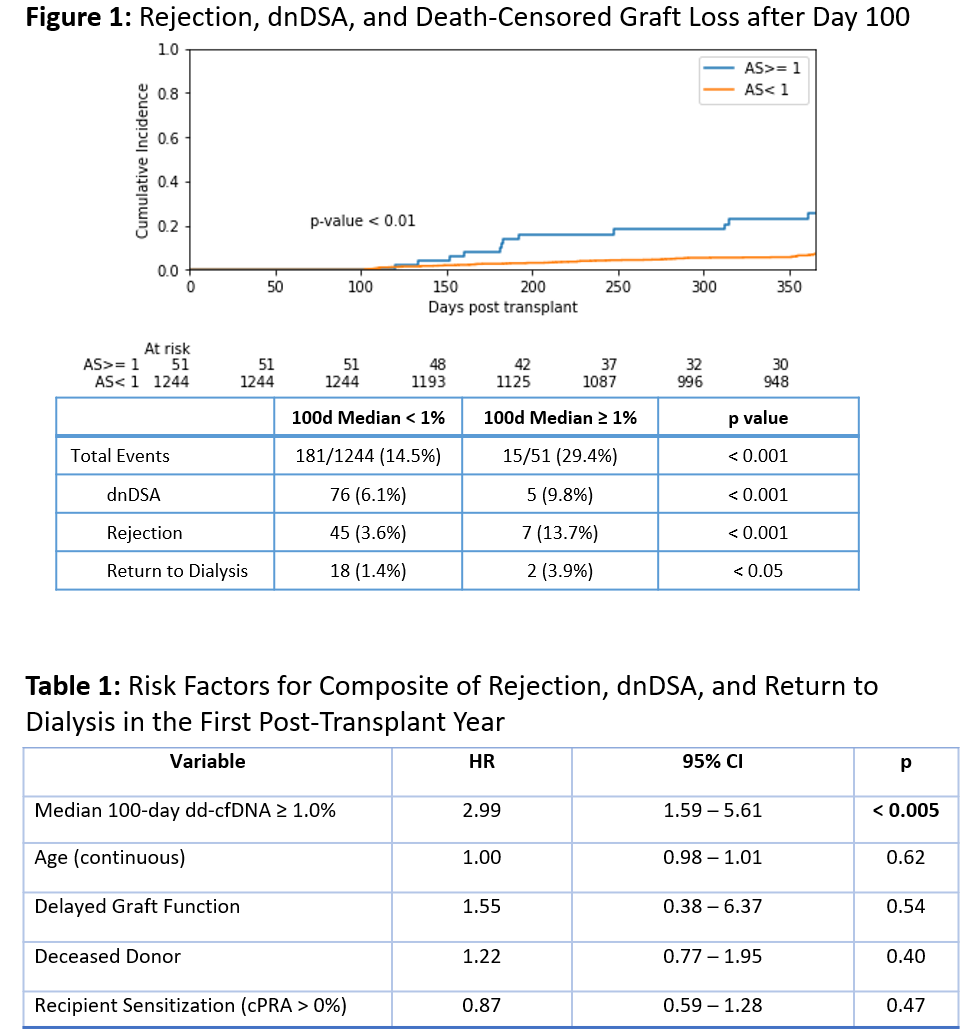
Conclusions: Our findings suggest that early post-transplant elevations in dd-cfDNA among kidney transplant recipients, even in the absence of clearimmunologic or histologic correlates, identify a population of patients at risk for adverse clinical outcomes during the first post-transplant year. Molecular risk-stratification using dd-cfDNA may have implications for clinical surveillance and therapeutic management of these patients.
