Hisashi Hashimoto, United Kingdom has been granted the TTS International Transplantation Science Mentee-Mentor Awards
Mitochondrial complex V inhibition enhances human regulatory T cell suppressive function through the induction of immunosuppressive small extracellular vesicles
Hisashi Hashimoto1, Joanna Hester1, Fadi Issa1.
1Nuffield department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
Translational Research Immunology Group (TRIG).
Introduction: Regulatory T cells (Tregs) have a critical role in immune tolerance. Clinical trials of autologous in vitro expanded Tregs therapy have demonstrated promising preliminary results, but there remain challenges to overcome including the donor-dependent in vitro expansion potential and time required for preparation. Here, we revealed that mitochondrial complex V inhibition enhances Treg suppressive function through the induction of immunosuppressive small extracellular vesicles which act in donor-independent manor.
Methods: Human CD4+CD25+CD127lo/- Tregs were flow sorted from peripheral blood mononuclear cells of healthy donors and expanded for two weeks in vitro in the presence of anti-CD3/anti-CD28 beads. Expanded Tregs were pre-treated by mitochondrial complex V inhibitor prior to the in vitro suppression assay to assess their suppressive function. To assess immunosuppressive soluble molecules, Treg culture supernatants were collected. To further identify these molecules, 100kDa ultracentrifugation filter and small extracellular vesicles depletion kits were applied to the supernatant prior to adding to T cell proliferation assay.
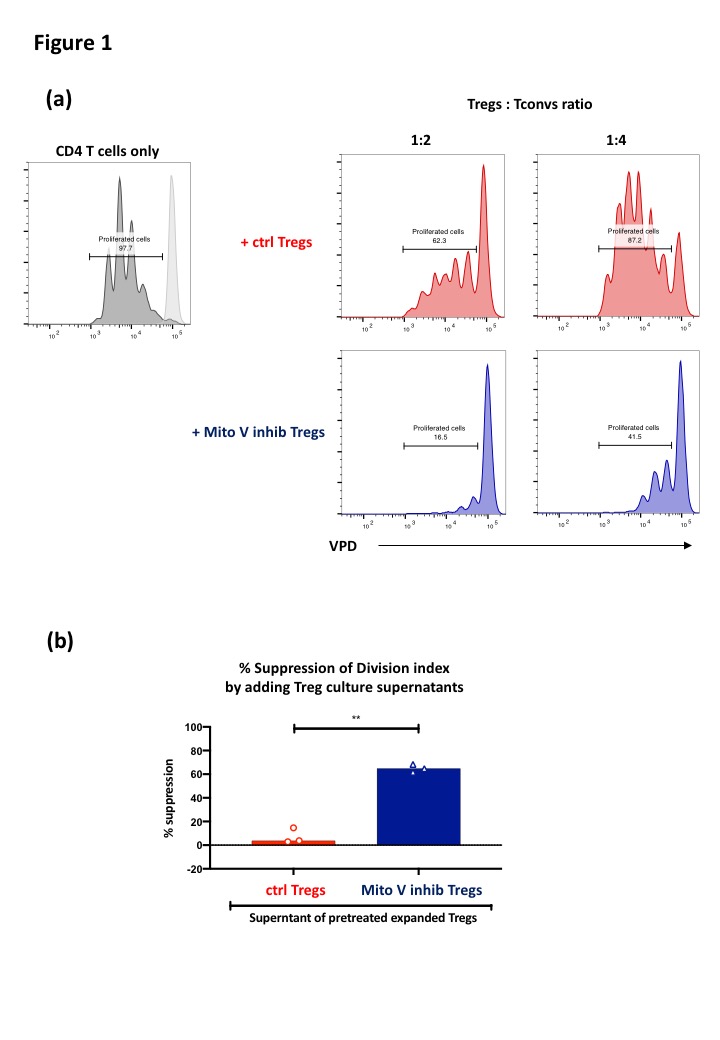
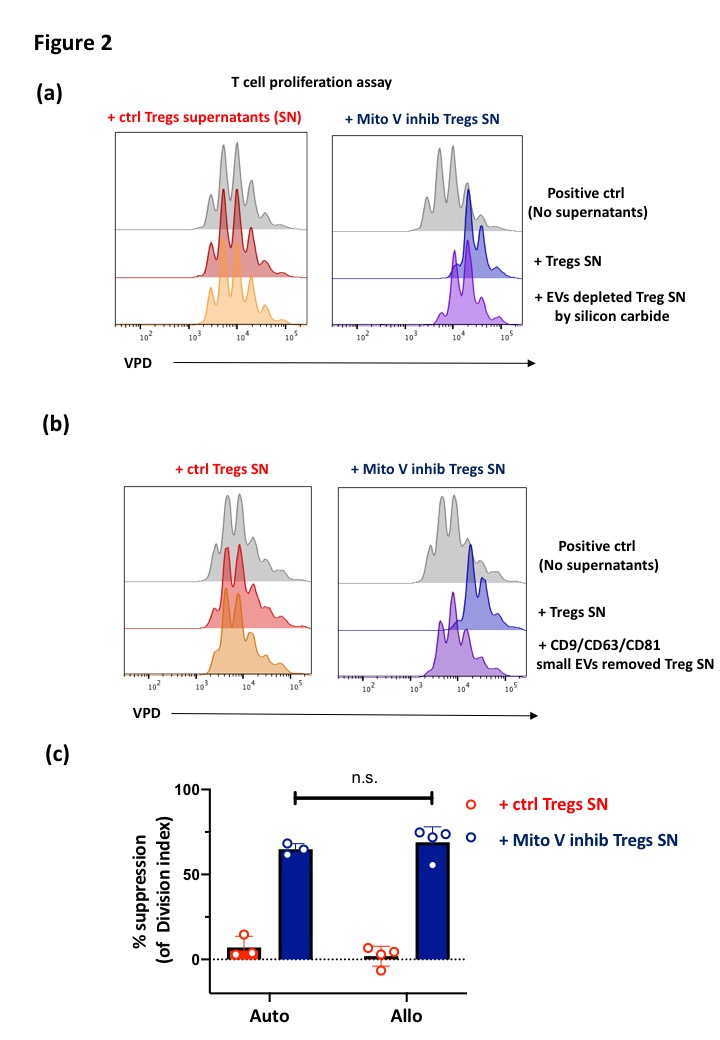
Results and Discussion: Mitochondrial complex V inhibitor pretreatment dramatically enhanced Treg suppressive function (Fig 1.a). However, pretreated Tregs did not increase their expression of known immune regulatory markers such as CTLA-4, PD-1, CD39 and CD25. We therefore hypothesized that pretreated Tregs produced immunosuppressive soluble molecules. Confirming this, Treg culture supernatants possessed strong suppressive function (Fig 1.b). Whilst pretreated Tregs produced higher levels of IL-10, only the >100 kDa fraction of the culture supernatant showed strong suppressive potential, indicating that the responsible suppressive factor was likely to be extracellular vesicles. Removal of small extracellular vesicles using either silicon carbide-based exosome removal kit (Fig 2.a) or anti-CD9, CD63 and CD81 magnetic beads (Fig 2.b) diminished the immunosuppressive properties of the pretreated Tregs culture supernatants, directly proving that Tregs produce immunosuppressive small extracellular vesicles after mitochondrial complex V inhibition. Lastly, we revealed that these immunosuppressive small extracellular vesicles suppressed 3rd party T cell proliferation (Fig 2.c).
Conclusions: This study reveals that pretreatment of pharmacological mitochondrial V complex inhibition can be used to enhance in vitro expanded Tregs function to reduce the required dose in Treg cellular therapy. Moreover, the donor-independent function of the small extracellular vesicles derived from mitochondria complex V inhibitor-treated Tregs may provide a new potential therapeutic strategy in which small extracellular vesicles could be used ‘off-the-shelf’.
