External Validation of the Toulouse-Rangueil Predictive Model to Estimate Donor Renal Function after Living Donor Nephrectomy
Manuela Almeida1,2, Jorge Malheiro1,2, Gonçalo Cruz4, Ciria Sousa3, Cátia Figueiredo5, Sofia Ventura1, José Silvano1, Sofia Correia1, Sofia Pedroso1, LaSalete Martins1,2.
1Nephrology, Centro Hospitalar Universitário do Porto, Porto, Portugal; 2Unit for Multidisciplinary Research in Biomedicine , Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto (ICBAS-UP), Porto, Porto, Portugal; 3Nephrology, Centro Hospitalar de Trás-os-Montes e Alto Douro (CHTMAD) - Vila Real, Portugal, Vila Real, Portugal; 4Nephrology, Hospital Garcia da Orta, Almada, Portugal; 5Nephrology, Centro Hospitalar do Médio Tejo, Torres Novas, Portugal
Background: Living donor kidney transplantation (LDKT) is the best treatment for end-stage kidney disease (ESKD). Long term follow-up data of the donors are reassuring but some donors will develop chronic kidney disease (CKD) and, rarely, ESKD. Precise tools to define these risks are lacking.A predictive model to estimate the 1-year post-donation glomerular filtration rate (eGFR) and risk of CKD was developed from a Toulouse-Rangueil cohort in 2017[1] and has been shown to have good correlation to the observed 1-year post-donation eGFR[2, 3].We aimed to externally validate this predictive tool in the a cohort of patients who underwent LDKT at our center.
Methods: Retrospective analysis of the 364 LKD at our center from 1998 to 2020. After exclusion of 33 donors, in whom eGFR at 1-year was missing, the remaining 333 donors were included in this study. Observed eGFR using CKD-EPI formula at 1-year post-donation was compared to the predicted eGFR using the formula developed in Toulouse-Rangueil: postoperative eGFR (CKD-EPI, mL/min/1.73m2) = 31.71 ± (0.521 × preoperative eGFR) – (0.314 × age). The ability of this formula to predict the observed GFR was analyzed by Pearson correlation, agreement was evaluated by the Bland-Altman plot and discriminative ability to predict CKD3-5 by the area under the receiver operating characteristic (ROC) curve and by plotting calibration.
Results: Patients’ characteristics of the 333 LKD are shown in table 1. Mean donor age was 47.3±10.6 years-old and most were female (71%).. A good correlation (Pearson r = 0.67; P < 0.001) and concordance (Bland-Altman plot with mean difference of observed-predicted eGFR = +2.33 mL/min/1.73 m2; 95% limits of agreement -21.41 to 26.47 mL/min/1.73m2;P < 0.001) were seen between predicted and observed 1-year post-donation eGFR.
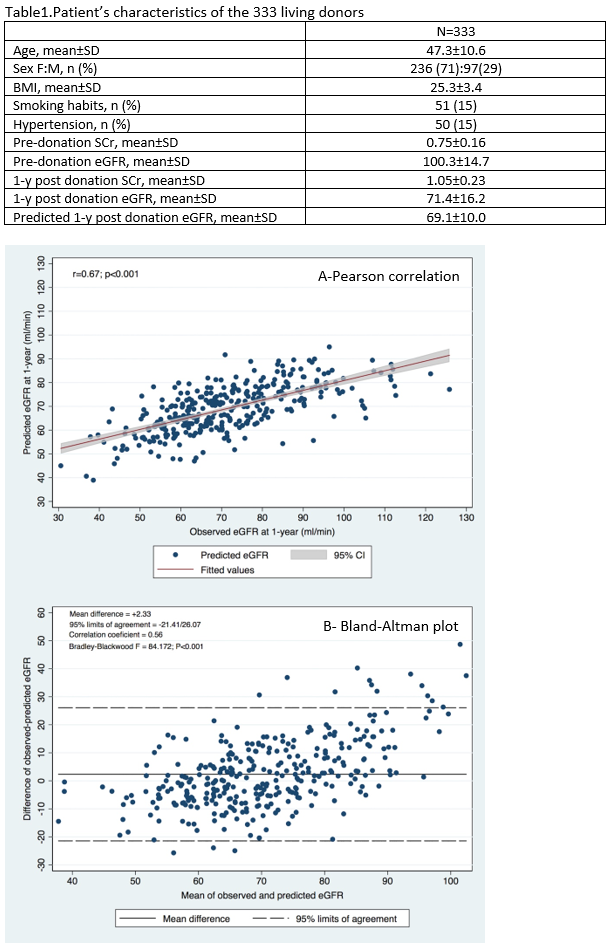
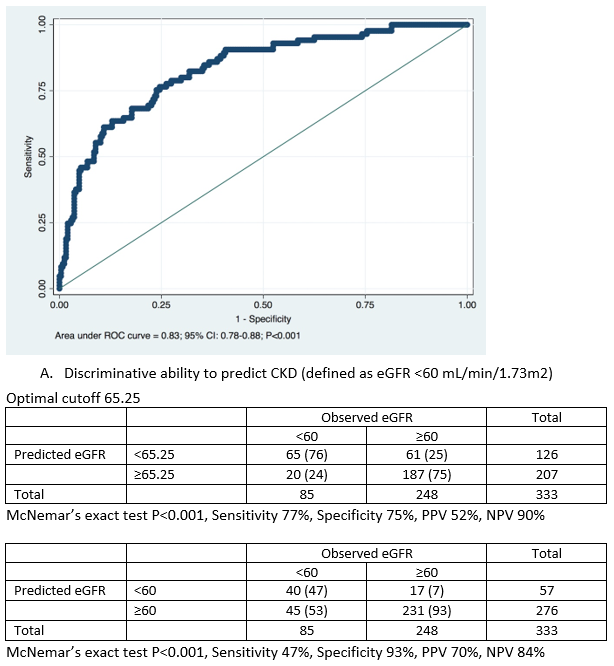
Area under ROC curve (AUC) showed a good discriminative ability of the formula in predicting observed CKD at 1-year post-donation (AUC = 0.83; 95% CI: 0.78-0.88; P<0.001), as shown in Figure 2, with optimal cutoff corresponding to a predicted eGFR of 65.25.7 mL/min/1.73 m2 (5.25ml above the equality cutoff),, for which the sensibility and specificity to predict CKD were respectively 77% and 75%.
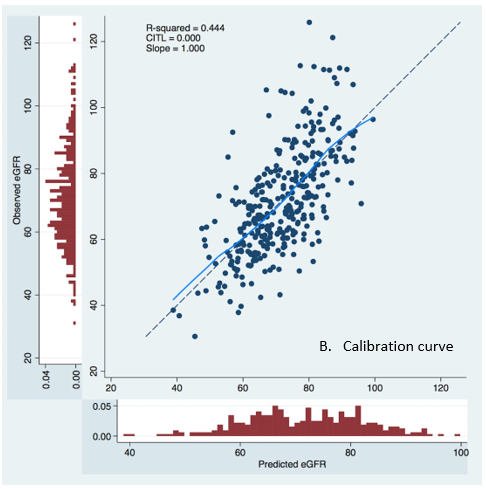
Calibration curve, shown in Figure 3, exhibited an excellent prediction with slope = 1.000 and CITL = 0.000.
 .
.
Conclusions: The formula developed in Toulouse-Rangueil was successfully validated in our cohort, a different European population than previous described [2, 3]. We must, anyway, emphasize that the optimal value of predicted eGFR was around 5 ml/min higher than the equality cutoff for CKD3-5 detection at 1-year, an outcome that was correctly predicted (both its presence or absence) in every 3 out of 4 donors. This model represents a simple and accurate tool that may be used to assist in the evaluation of potential donors, particularly in the setting of current increasing donor age.
