Postoperative statin therapy is not associated with reduced incidence of venous thromboembolic events following kidney transplantation
Peter Frasco1, David Rosenfeld1, Caroline C Jadlowiec2, Nan Zhang3, Raymond L Heilman4, Karl A Poterack1.
1Anesthesiology, Mayo Clinic Arizona, Phoenix, AZ, United States; 2Transplant Surgery, Mayo Clinic Arizona, Phoenix, AZ, United States; 3Quantitative Research, Mayo Clinic Arizona, Phoenix, AZ, United States; 4Transplant Nephrology, Mayo Clinic Arizona, Phoenix, AZ, United States
Background: Deep vein thrombosis (DVT) and pulmonary embolism (PE), collectively venous thromboembolism (VTE), are common sources of morbidity and mortality following solid organ transplantation. The pleiotropic effects of statin therapy on inflammation and coagulation may reduce the risk of venous thromboembolism. This study evaluated whether statin therapy is associated with decreased venous thromboembolic (VTE) events following kidney transplantation.
Methods: We performed a retrospective analysis of all primary kidney transplants performed between January 2014 and December 2019 at Mayo Clinic Arizona. Patients were divided into two groups depending on sustained statin therapy during the first year following transplantation. Statin exposure was defined as any statin prescribed on admission for KT or upon discharge from KT plus continous treatment for one year. Recipient and donor clinical and demographic data were collected. The primary outcome was admission for symptomatic VTE events (deep vein thrombosis (DVT) or pulmonary embolism (PE)). Cox regression was used to model overall survival and VTE event-free survival. Fine and Gray's regression was used to model outcome for VTE while treating death as a competing risk event.
Results: Sustained statin therapy in the first year following transplant was observed in 16.1% (n=223) of 1384 kidney transplants included in the study cohort. The control group of 1161 patients had no statin exposure during the first year following KT. Demographic and perioperative characteristics are presented in Table 1. The overall incidence of VTE events in the year following kidney transplant was 3.8%. VTE occurred in 4.1% of recipients treated with statins and 3.8% of the controls – (hazard ratio [HR] 0.96, 95% confidence interval [95% CI] 0.43, 2.14, P = 0.93) (Table 2).
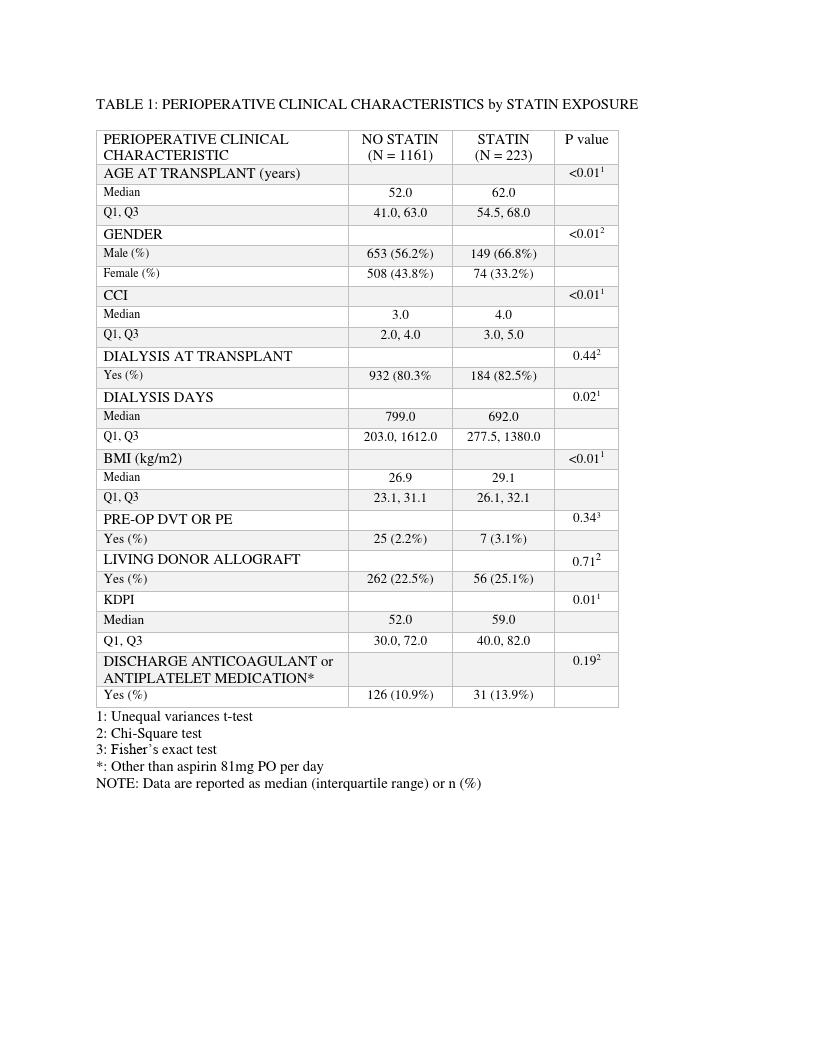
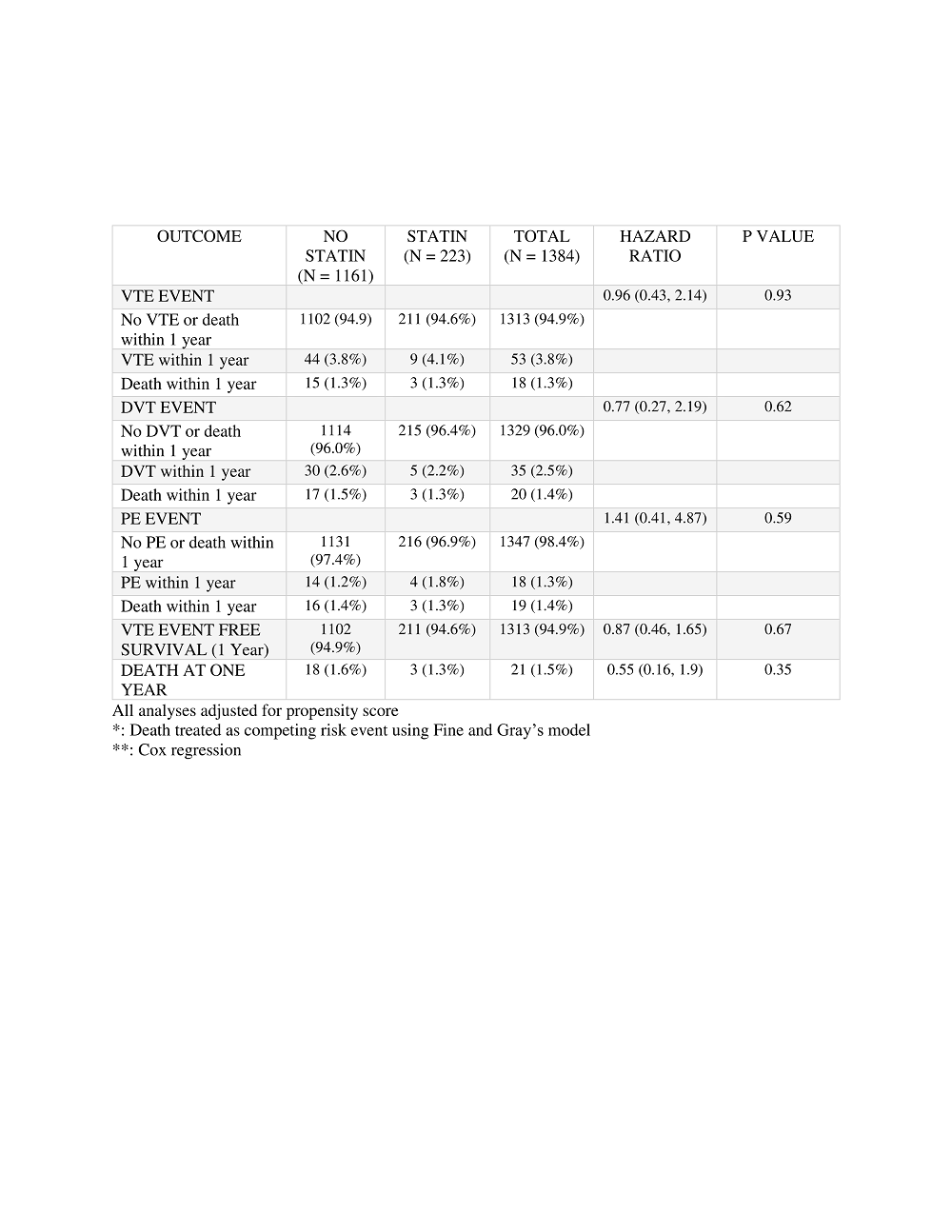
However, there were significant differences between the groups in terms of age, gender and body mass index. Following sensitivity analysis in which cohort matching was performed to account for these differences, there was no difference in VTE event-free survival (HR 0.85, 95% CI 0.43, 1.68, P=0.65) or overall survival (HR 0.54, 95%CI 0.15, 1.94, P= 0.35) between patients treated with statins compared to controls (Figure 3).
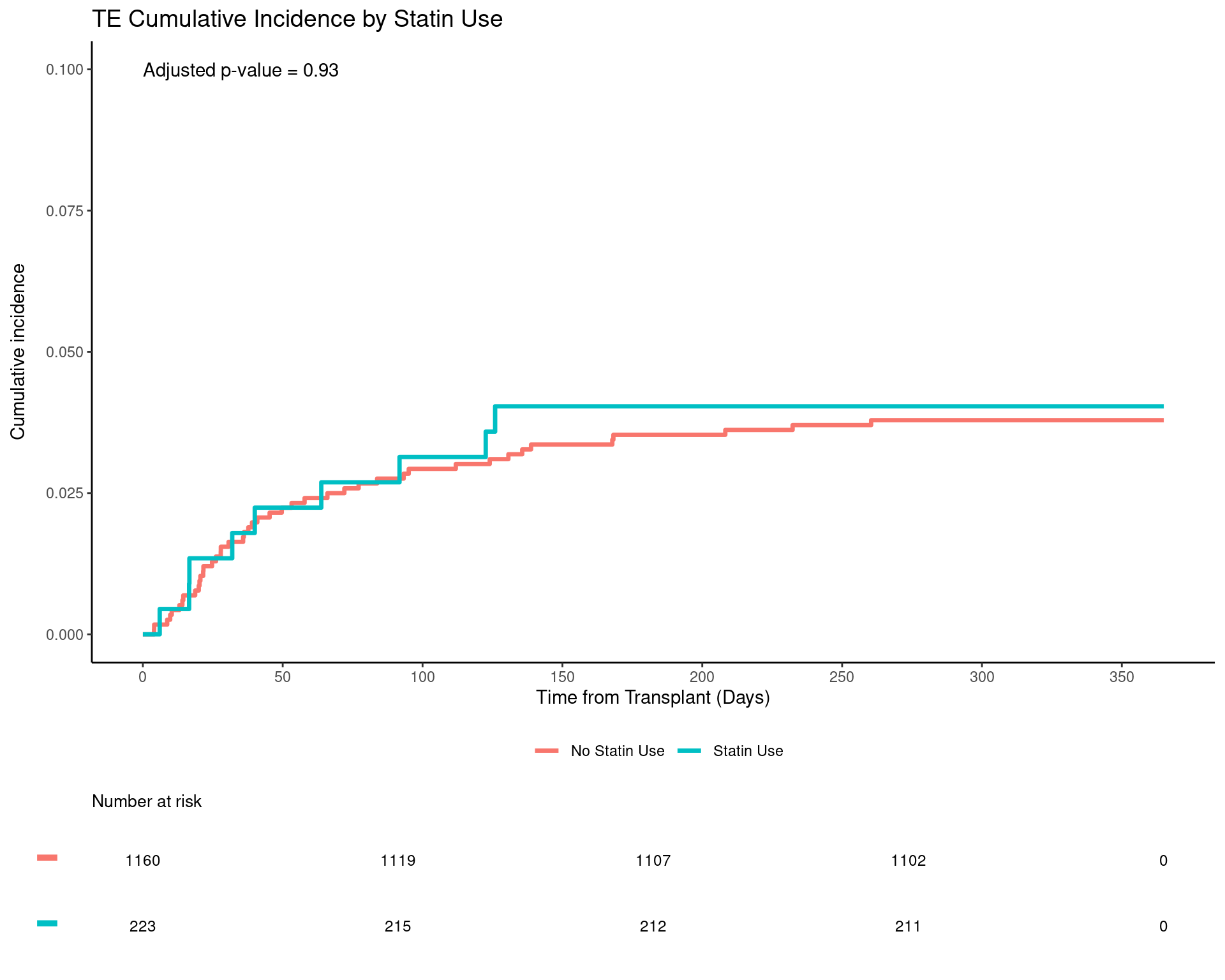
Conclusion: In this single center, retrospective investigation, statin therapy in the year following successful kidney transplant was not associated with a reduction in risk of VTE. Although previous investigations have demonstrated ameliorative effects of statin therapy on rejection, VTE, cancer recurrence and vasculopathy following liver, heart and lung transplantation, the current study and others have failed to confirm any such benefit in the kidney transplant population.
