Metabolic characterization of chronic antibody-mediated rejection (CABMR) using NMR-based serum metabolomics
Mantabya Singh1, Durgesh Dubey3, Narayan Prasad1, Vikas Agarwal2, Dinesh Kumar3.
1Nephrology, SGPGIMS, Lucknow, India; 2Clinical Immunology, SGPGIMS, Lucknow, India; 3Center for Biomedical Research (CBMR), Lucknow, India
Background: A common complication after renal transplantation is allograft rejection, which often leads to chronic rejection and eventual graft loss. While renal allograft biopsy continues to be considered the gold standard in the diagnosis of chronic rejection. The development of non-invasive methods for the accurate detection of chronic rejection of renal grafts has thus become of important clinical importance.
Methods: NMR-based serum metabolomics was employed for analysis of serum metabolites in 18 renal allograft recipients with chronic rejection and 28 with non-chronic rejection. Samples were analyzed by an 800 MHZ NMR spectrometer. The metabolic profiles and differential metabolites of sera were analyzed by multivariate statistical analysis (MSA), including orthogonal partial least squares discriminant analysis (OPLS-DA) methods.
Results: The orthogonal projection to latent structures discriminant analysis (OPLS-DA) model resulted in an R2(Cum) of 0.9 and a Q2 (Cum) of 0.5 for CABMR and NCABMR subjects, respectively. Among the differential unregulated metabolites identified in CABMR, citrate, acetyl-carnitine, carnitine, proline, and tyrosine were upregulated from MSA. Citrate had the highest discriminatory potential (AUC 0.8, P=0.0006) followed by carnitine (AUC 0.7, P=0.02, and proline (AUC 0.7, P=0.01). The results demonstrated that CABMR possesses an active Phenylalanine, Tyrosine, and Tryptophan synthesis pathway.
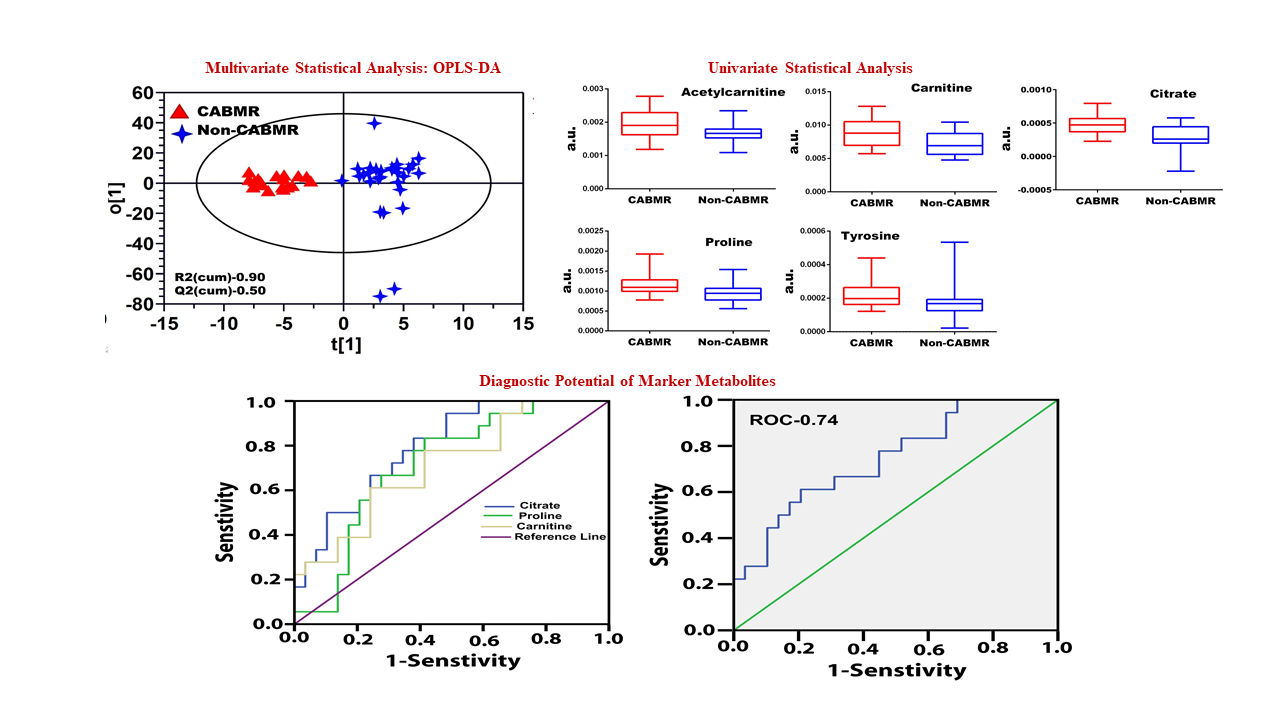
Conclusions: Despite being in its early stages, metabolomics monitoring in kidney transplantation can provide reliable indicators of chronic kidney injuries and allograft rejection The diagnostic model that evolved in this study may prove valuable as a tool for a definitive diagnosis of CABMR and NCABMR patients after validation in larger sample sizes.
