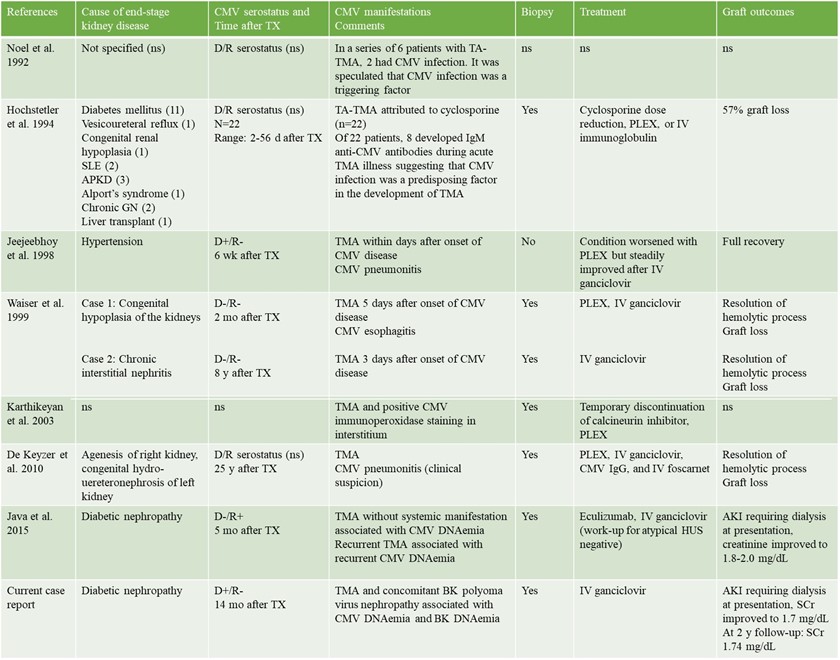
Cytomegalovirus-induced thrombotic microangiopathy after renal transplant: case report and review of the literature
Mrinalini Sarkar1, Justin Oveyssi1, Jonathan E Zuckerman2, Phuong-Chi T Pham3, Phuong-Thu T Pham1.
1Division of Nephrology, UCLA -David Geffen School of Medicine, Los Angeles, CA, United States; 2UCLA Department of Pathology and Laboratory Medicine, UCLA -David Geffen School of Medicine, Los Angeles, CA, United States; 3Division of Nephrology, UCLA Olive View Medical Centre, Sylmar, CA, United States
Introduction: CMV infection as a triggering factor for de novo post-transplantation TMA has long been suggested. We herein report a case of CMV and BKPyV co-infection in a kidney transplant recipient and CMV-induced TMA manifested by severe AKI and thrombocytopenia.
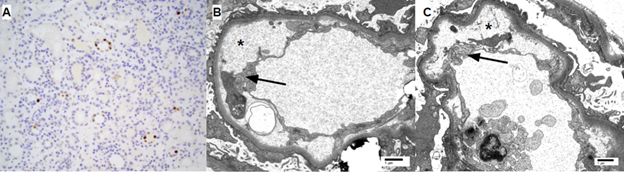
Case report: A 48-year-old man with end stage kidney disease secondary to diabetes mellitus underwent a 4 HLA-mismatched, deceased donor renal transplant from a 40-year-old donor who died of a cerebrovascular accident. Cold ischemia time and warm ischemia time were 31 and 18 minutes respectively. CMV IgG was positive in donor and negative in recipient. Intravenous methylprednisolone and antithymocyte globulin were used for induction for anticipated delayed graft function. He was discharged on postoperative day 6 with a serum creatinine (SCr) of 8.1 mg/dL.. His allograft function continued to improve, achieving a SCr of 1.67 mg/dL at 1-month post-transplantation. His SCr had since fluctuated between 1.4-1.7 mg/dL. At 4-month post-transplantation, he was found to have low grade BK DNAemia (900 copies/mL) managed with mycophenolate dose reduction alone. He did well until 14-month post-transplantation when he presented with non-oliguric AKI with a SCr of 15.6 mg/dL (baseline 1.5 mg/dL) requiring dialysis initiation. Peripheral smear demonstrated thrombocytopenia but no schistocytes. A renal allograft biopsy (figure 3) was performed which demonstrated BK nephropathy, TMA, and no evidence of rejection.Labs revelaed CMV DNAemia of >140,000 copies/ml and BK DNAemia of 17,000 copies/ml. Donor-specific antibodies, HIV RNA polymerase chain reaction (PCR), hepatitis C RNA PCR, anticardiolipin antibody, stool shiga toxin PCR, and stool and blood cultures were all negative.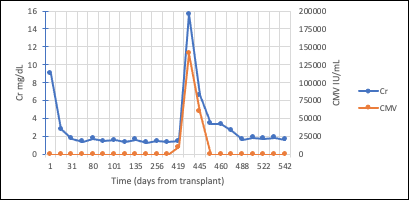
Intravenous ganciclovir was initiated with improvement in CMV DNAemia and concomitant improvement in renal function. He was transitioned to oral valganciclovir after 10 days of intravenous therapy with complete resolution of CMV DNAemia and steady improvement in renal function. His SCr continued improve and nadired at 1.7 mg/dL. At 2-year follow-up, his SCr was 1.74 mg/dL.
Discussion: Initiation of IV ganciclovir and reduction in CMV DNAemiwith decrease in SCr and a transition of the patient off hemodialysis suggested that CMV is the causative factor for TMA. CMV-induced renal injury can manifest as various histopathological patterns, commonly as a CMV glomerulopathy with viral inclusions usually seen in the glomerular endothelial cells, acute tubulointerstitial nephritis and rarely as TMA.Improvement in renal function despite continuation of tacrolimus in current patient suggested that calcineurin-inhibitor-induced TMA was unlikely.Although the precise mechanism whereby CMV viremia may cause TMA is currently not fully understood, it is speculated that infection can initiate endothelial cell damage and inflammation which triggers the thrombotic cascade leading to TMA.