
Steroid avoidance and progression of interstitial fibrosis assessed by automated quantification after kidney transplantation: a multicenter randomized non-Inferiority trial (the ASTRONEF study)
Simon Ville1, Vannary Meas-Yedid2, Morgane Pere3, Diego Cantarovich1.
1Institute of Transplantation-Urology-Nephrology, Nantes University Hospital, Nantes, France; 2BioImage Analysis Unit, Pasteur Institute, Paris, France; 3Biostatistique Unit, Nantes University Hospital, Nantes, France
ASTRONEF Study Group.
Introduction: Corticosteroid avoidance has emerged as a standard of care in the low-immunological risk kidney transplant recipients as it does not increase the risk of acute rejection. However, another concern is the 1-year post-transplantation onset of interstitial fibrosis (IF) that could be mitigated by steroids. In that randomized clinical trial, we addressed whether the absence of corticosteroids impacted the progression of the fibrosis, assessed at implantation and at 1-year post-transplantation.
Methods: Adult recipients, of a renal allograft from a non– HLA-identical living or deceased donor were randomly assigned to receive on top of basiliximab, advagraf and MMF, steroid-free regimen (-CS group) or a standard corticosteroid tapering regimen (+CS group): 20 mg of oral prednisone on day 2 through 14, 15 mg on days 15 through 30, 10 mg on days 31 through 90, then from day 91, 5mg until day 365. Protocol biopsy was performed at implantation then at 3 and 12 months and interstitial fibrosis (IF) was assessed with a validated quantitative and automated method and expressed as a percentage. The primary endpoint was the difference in percentage change in IF between the implantation and the 1-year biopsy, with a hypothesis of non-inferiority of 10%.
Results: One hundred and eight patients were analyzed in the full analysis set: 52 patients in the +CS group and 56 in the - CS one. Complete avoidance of corticosteroids was reached in 36 (64%) -CS patients that make up, along with all the 52 +CS patients, the per-protocol (PP) population. Figure 1 illustrates the distribution of the percentage of IF at each time points according to the treatment group. In the FAS population, mean percentage of IF at implantation was 19.5(±7.9)% in the -CS group (n=51) and 17.9(±7.8)% in the +CS group (n=49) (p = 0.3) and 25.9(±11.0)% (n=43) vs 21.5(±11.2)% (n=39) (p = 0.03) at 1-year. The progression of IF between the biopsy at implantation and at 1-year was of 7(±13.1)% (n=42) and 4.2(±11.5)% (n=37) in the -CS group and + CS group respectively (p = 0.3). When considering the difference, the steroid-free regimen was non-inferior 3.5% IC 95% [-0.5%; 7.5%]. In the PP population the progression of IF in the -CS group was of 4.2(±12.8)% and also steroid-free regimen was non-inferior (2.0% IC 95% [-2.7% ; 6.7%]). By 1 year after transplantation, biopsy-proven acute rejection occurred in 4(7.1%) patients of -CS group versus 2(3.8%) of +CS group (p = 0.7).
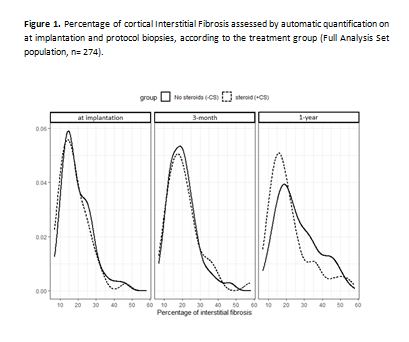
Conclusion: In a randomized multicenter clinical trial, we found that steroid avoidance in kidney transplant recipients with low immunological risk, was non-inferior regarding the progression of interstitial fibrosis during the first-year post-transplantation assessed with a quantitative metric.
