Patient and graft survival after A1/A2-incompatible living donor kidney transplantation
Shivani Bisen1, Samantha N. Getsin1, Po-Yu Chiang1, Kayleigh Herrick-Reynolds1, Laura B. Zeiser1, Sile Yu1, Niraj M. Desai1, Fawaz Al Ammary2, Kyle Jackson1, Dorry L. Segev1,3,4, Allan B. Massie1,3.
1Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 2Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States; 3Department of Epidemiology, Johns Hopkins University School of Public Health, Baltimore, MD, United States; 4Scientific Registry of Transplant Recipients, Minneapolis, MN, United States
Epidemiology Research Group in Organ Transplantation.
Background: ABO type B and O kidney transplant candidates often have increased difficulty identifying a compatible donor for living donor kidney transplantation (LDKT) and are harder to match in kidney paired donation (KPD) registries. A2-incompatible (A2i) LDKT increases access to LDKT for these patients. Kidney transplantation across the A2 barrier (A2 -> O, A2 -> B, A2B -> B) is believed to be safer than that across the A1 barrier (A1 -> O, A1 -> B, A1B -> B) because A2 kidneys express fewer A antigens on their renal endothelial surfaces. However, the differential risk of A2i LDKT and its relative benefit when compared to A1-incompatible (A1i) LDKT, if any, remains unknown. To better inform living donor selection, we evaluated the association between A2i LDKT and patient and graft survival.
Methods: We used inverse probability weighted Cox regression to compare mortality, death-censored graft failure, and all-cause graft loss in A2i vs. ABO-compatible (ABOc) recipients. These methods were repeated to compare mortality, death-censored graft failure, and all-cause graft loss between A1i vs. comparable ABOc LDKT recipients.
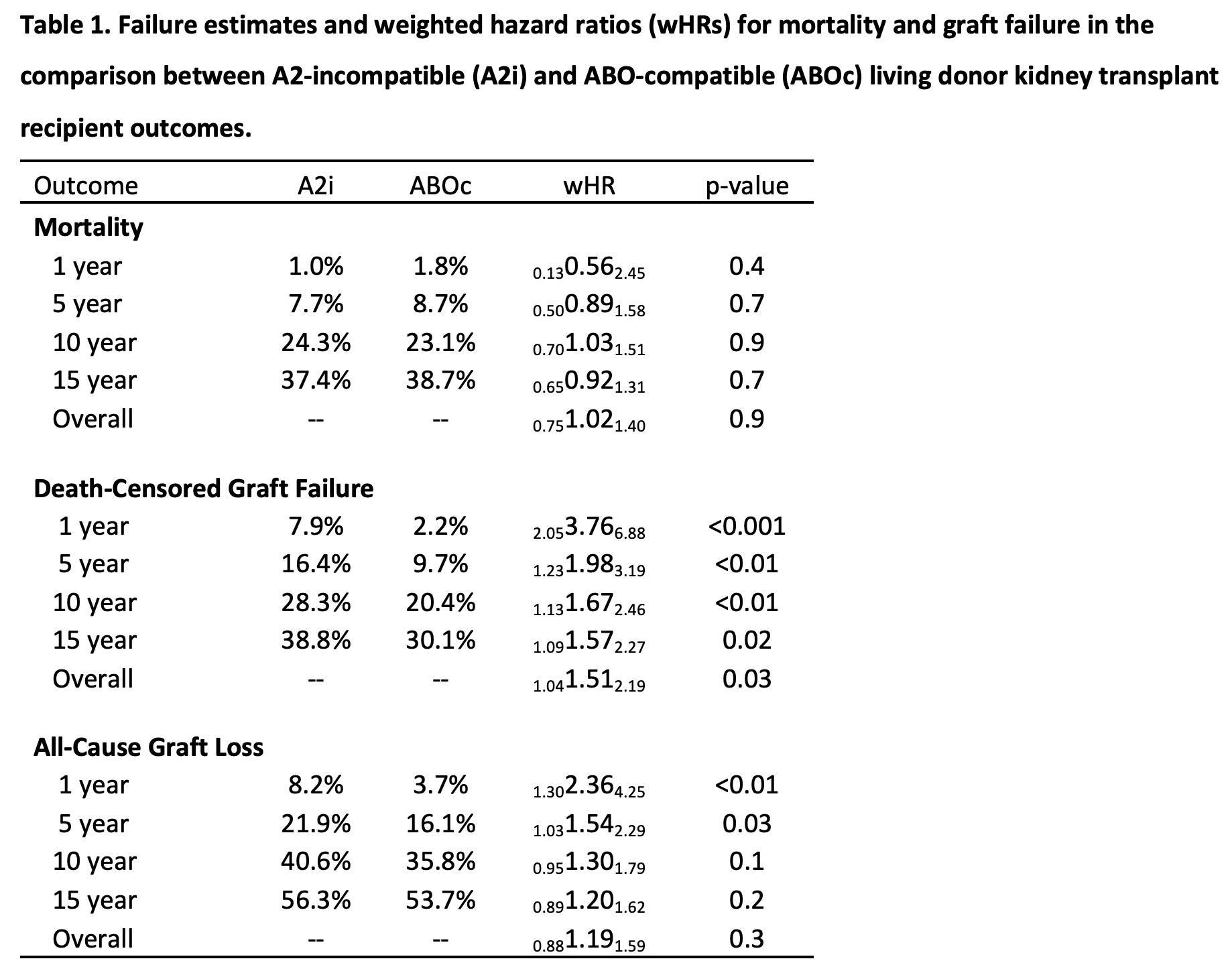
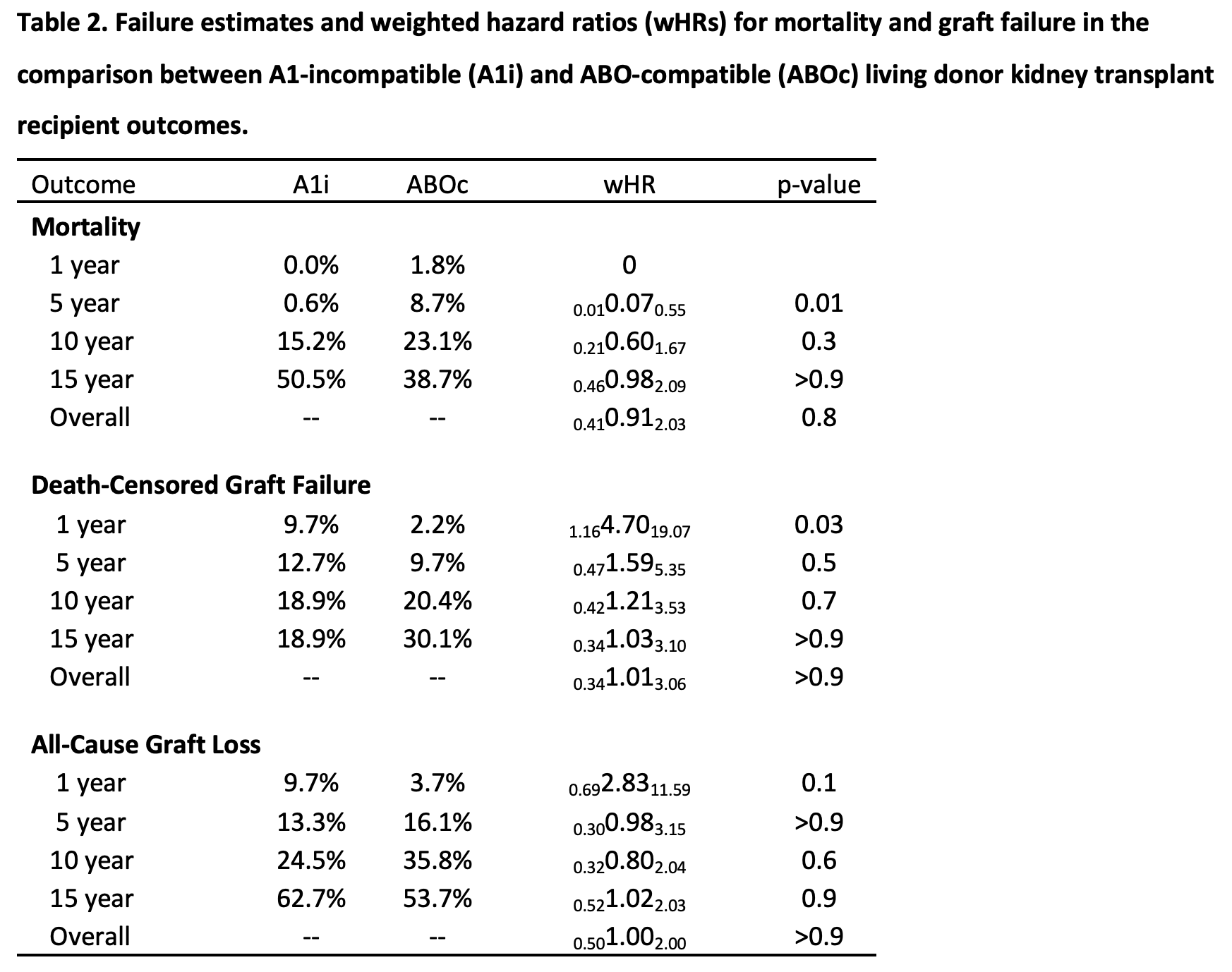
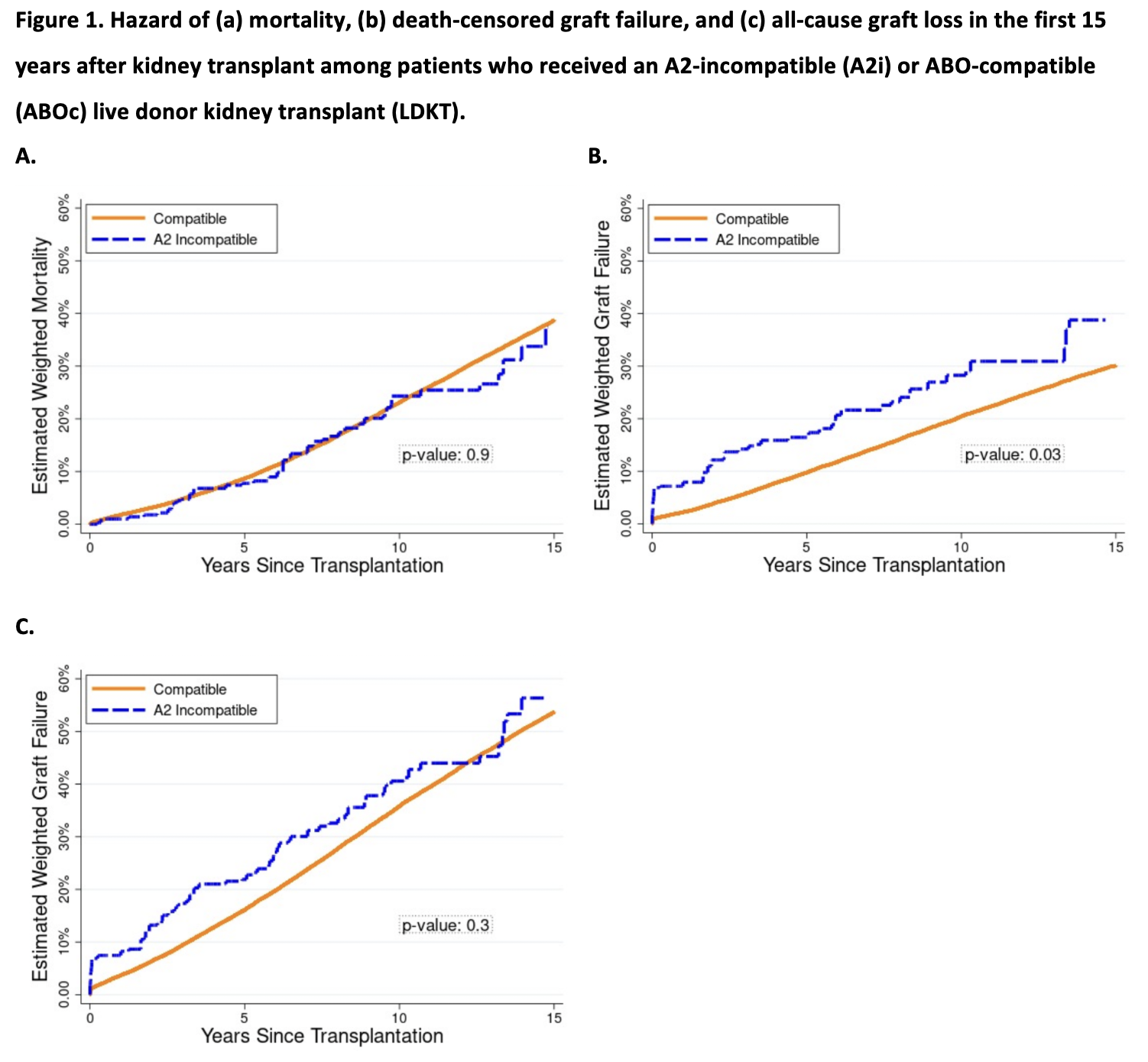
Results: Using data from the Scientific Registry of Transplant Recipients (SRTR) 2000-2019, we identified 345 A2i LDKT recipients. Mortality was comparable among A2i and ABOc recipients; weighted 1/5/10-year mortality was 1.0%/7.7%/24.3%, respectively, among A2i LDKT recipients vs. 1.8%/8.7%/23.1%, respectively, among comparable recipients in the ABOc LDKT weighted cohort (wHR 0.751.021.40, p=0.9). However, A2i recipients faced higher risk of death-censored graft failure; weighted 1/5/10-year graft failure was 7.9%/16.4%/28.3% for A2i vs. 2.2%/9.7%/20.4% for ABOc recipients (wHR in year 1 = 2.053.766.88; through year 5 = 1.231.983.19; through year 10 = 1.131.672.46). By comparison, 1/5/10-year weighted hazard ratios for death-censored graft failure among A1-incompatible recipients were 0.164.7019.07/0.471.595.35/0.421.213.53.
Conclusions: This registry analysis considered the differential risk of A2i LDKT in the United States and demonstrated comparable mortality to ABOc LDKT recipients, but an increased risk of death-censored graft failure for A2i LDKT recipients, particularly in the first year post-transplant. Nonetheless, A2i LDKT remains an excellent treatment option, providing access to LDKT for patients who must otherwise remain on the waitlist in hopes of receiving DDKT. Our findings further the field’s understanding of the risks and benefits of A2i LDKT, and should inform patient counseling, KPD matching algorithms, and post-operative monitoring procedures in the future.
K24AI144954 from the National Institute of Allergy and Infectious Diseases (NIAID). K01DK101677 from the National Institute of Diabetes andDigestive and Kidney Diseases (NIDDK).
