The gut microbiome facilitates metabolic syndrome (MBS) post kidney transplantation
Peter J. Mannon1, Jianguo Chen2, Amanda Pendergraft3, Steve Barnes4, Roslyn B. Mannon1.
1Medicine, University of Nebraska Medical Center, Omaha, NE, United States; 2Medicine, UAB, Birmingham, AL, United States; 3College of Public Health, UAB, Birmingham, AL, United States; 4Pharmacology, UAB, Birmingham, AL, United States
Introduction: Death with kidney allograft function remains a challenge due to the propensity of kidney transplant recipients (KTRs) towards cardiovascular comorbidities including the metabolic syndrome (MBS). This is in spite of efforts to modify these risks via immunosuppressive management and steroid avoidance. Dysbiosis of the gut microbiome impacts lipid metabolism, insulin resistance and atherosclerotic factors. Gut dysbiosis has been demonstrated in KTRs with possible links to the alloimmune response. We hypothesized that changes in gut microbiome pre- and post-transplant may contribute to the development of MBS in KTRs.
Methods: We prospectively collected stool specimens from 14 consecutive patients with End Stage Kidney Disease prior to receiving a living donor kidney transplant (pre-Tx), with a second collection at 6-12 months post-transplant (post-Tx). Eight healthy controls provided specimens for comparison. Demographics and clinical variables were extracted from the electronic medical record. Stool was analyzed for 16S rRNA gene sequencing, microbial metagenome analysis, and targeted and untargeted metabolomics. We measured 69 gut metabolites in serum (GC-TOFMS with a XCF [methyl and ethyl chloroformate] deviation method) and 104 fecal metabolites (LC-MS measures of bile salt and other metabolites). The primary outcomes were differences in the microbiome and metabolome of KTRs pre-Tx vs control and vs post-Tx. Secondary outcomes were microbiome and metabolome differences between pre-Tx versus post-Tx stratified by MBS status and if a microbiome or metabolome “signature” predicted MBS outcome in KTR.
Results: 11 KTRs met criteria for MBS pre-Tx, of which 8 were stable or worsened MBS and 3 improved MBS post-Tx. 1 patient developed MBS de novo. Compared to controls, pre-Tx gut microbiome were less diverse, had increased E.coli and Fusobacterium and less Coprococcus and Roseburia, had higher CKD metabolites, lower short chain fatty acids, and higher glutathione (oxidative stress) and proteolytic metabolic pathways. Post-Tx microbiome showed increased Roseburia and decreased Akkermansia, less uremic toxins and less proteolytic pathways. Untargeted metabolomics aligned with improvement in MBS post-Tx (Fig. 1) and independently discriminated between MBS outcomes (Fig. 2). Improved MBS post-Tx was associated with increased Ruminococcus, decreased Akkermsansia, and saccharolytic (and butyrogenic and methanogenic) pathways.
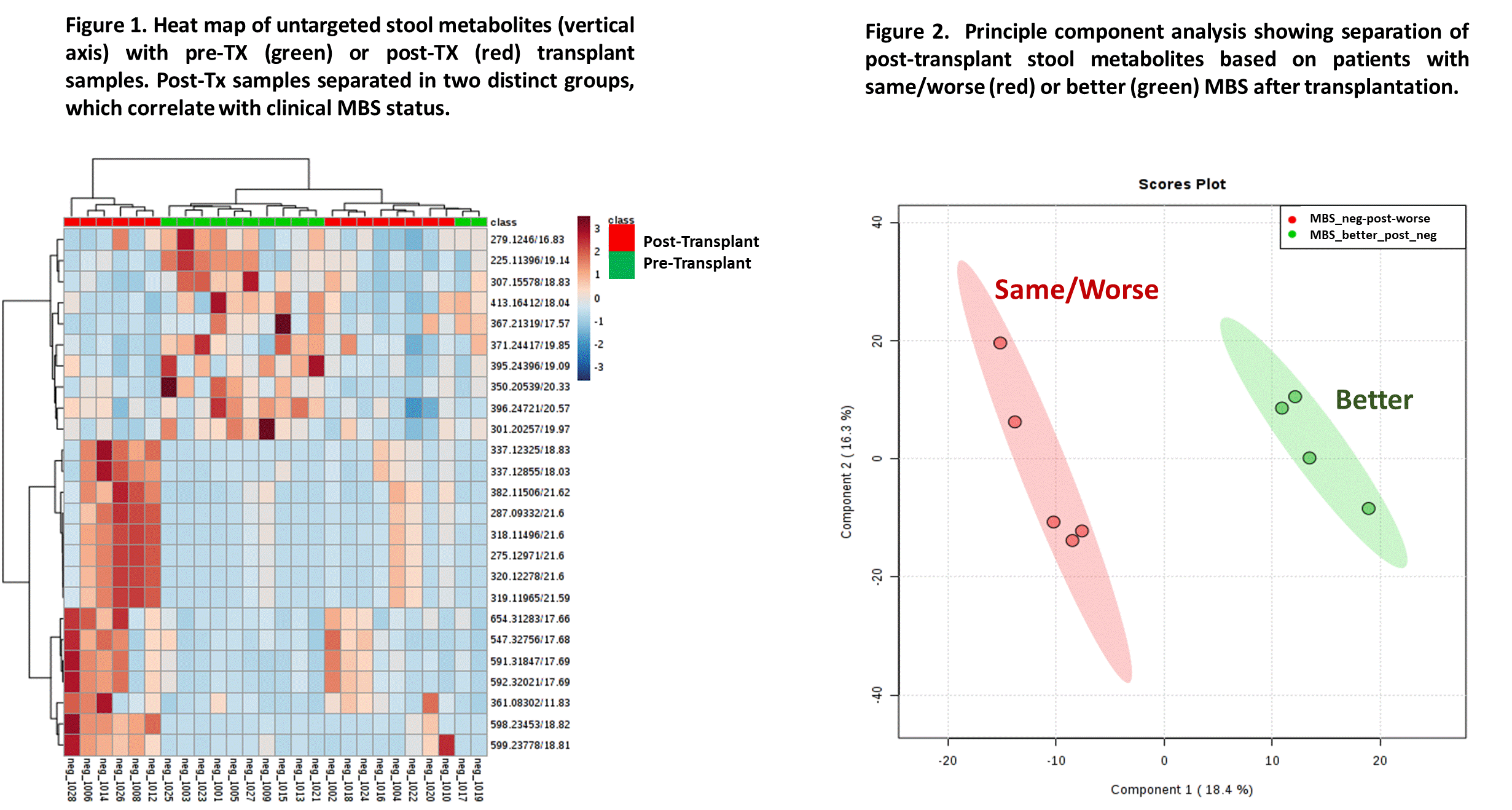
Conclusion: Our findings demonstrate that the gut microbiome can clearly discriminate pre- and post-kidney transplant MBS states and also provides a signature for status of MBS post-Tx. These data support efforts to condition the gut microbiome using targeted prebiotics and probiotics to confer a beneficial metabolic state pre-Tx and post-Tx. Gut microbiome manipulation may provide potential adjunct approach to support long term patient and graft survival.
