
High alemtuzumab exposure is associated with delayed lymphocyte recovery in kidney transplant recipients
Suzanne Bezstarosti1,2, Tom C. Zwart3, Federica R. Achini4, Marco W. Schilham4, Dirk Jan A. R. Moes3, Marlies E. J. Reinders2, Johan W. de Fijter2, Sebastiaan Heidt1,5.
1Immunology, Leiden University Medical Center, Leiden, Netherlands; 2Internal Medicine (Nephrology), Leiden University Medical Center, Leiden, Netherlands; 3Clinical Pharmacy and Toxicology, Leiden University Medical Center, Leiden, Netherlands; 4Paediatrics, Leiden University Medical Center, Leiden, Netherlands; 5Eurotransplant Reference Laboratory, Leiden, Netherlands
Background: While alemtuzumab is increasingly being used as an induction therapy in kidney transplantation, little is known about the relation between alemtuzumab exposure, lymphocyte recovery and clinical outcomes in kidney transplant recipients (KTRs). Recently, a population pharmacokinetic model was developed based on alemtuzumab plasma concentrations of KTRs in the Triton Study, which allows for estimation of individual alemtuzumab pharmacokinetics. We hypothesized that alemtuzumab exposure would demonstrate substantial interpatient variability and correlate with lymphocyte recovery rate and infection incidence post-transplantation (post-Tx).
Methods: KTRs in the control arm of the Triton Study (n=29) were included in this analysis. All patients received alemtuzumab induction (2x 15 mg subcutaneously), steroids, tacrolimus and everolimus. As previous studies have suggested that alemtuzumab has lympholytic capacity at concentrations exceeding 0.1 mg/L, the time above this threshold concentration (TATC) and the maximum concentration (Cmax) were predicted using a validated population pharmacokinetic model. Immune monitoring of lymphocyte subsets was performed at baseline and at week 6, 12, 24, 52 and 104 post-Tx. CD4 T cell recovery was defined as > 50,000 CD4 T cells/ml peripheral blood, as counts below this limit have been associated with a higher risk of viral reactivation.
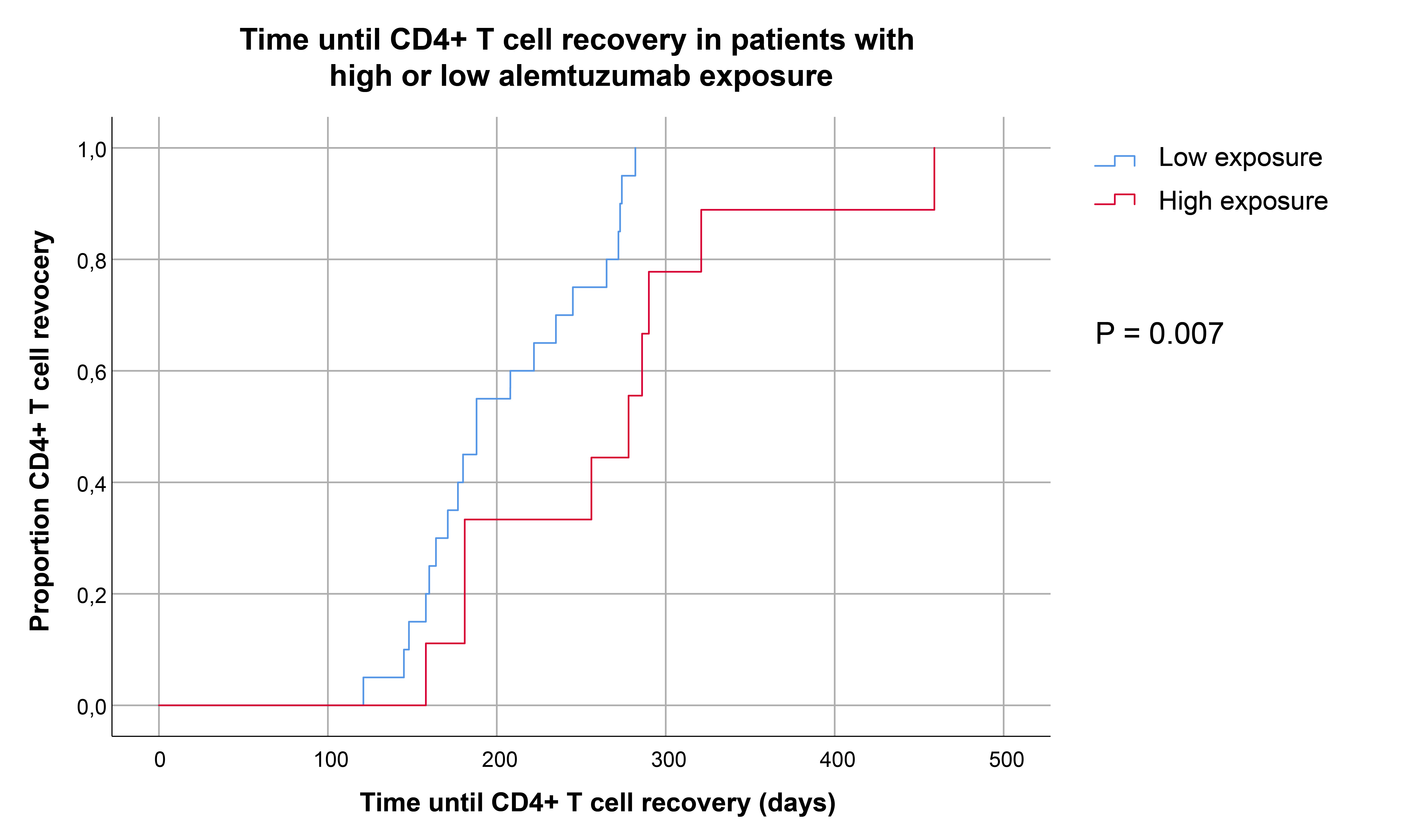
Results: The predicted TATC and Cmax demonstrated considerable interpatient variability ranging from 29.9-64.0 days (median: 39.7) and 0.54 to 1.39 mg/L (median: 0.80) respectively. KTRs with a TATC > 66th percentile (42.4 days) were categorized as ‘high exposure’ and patients below this threshold as ‘low exposure’. Median absolute CD4 T cell numbers at week 6 (118 vs 443, P=0.0096) and week 12 (1519 vs 7762, P=0.003) were lower in KTRs with high exposure than in patients with low exposure. Similar trends were observed for CD8 T cells, regulatory T cells and to a lesser degree for B cells. In order to predict the timepoint that patients reached CD4 T cell recovery more accurately, time to CD4 T cell recovery for every patient was predicted using a basic, empirical nonlinear mixed effects model. Kaplan Meier analysis demonstrated that KTRs with high exposure reached CD4 T cell recovery at later timepoints than patients with lower exposure (Figure 1). Regardless of differences in lymphocyte recovery rate, there was no statistically significant difference in the cumulative incidences of BK-virus, CMV, EBV or de novo donor-specific antibody formation between the two groups.
Conclusions: Alemtuzumab exposure demonstrates extensive interpatient variability and impacts lymphocyte recovery rate early after transplantation. Personalized dosing strategies could possibly limit interpatient variability in alemtuzumab exposure and subsequently lymphocyte recovery rate, but further research is warranted to study the potential clinical benefit.
