Liver retransplantation in adults: indications and outcomes - analysis of a 23-year experience in a single center in Argentina
Victoria Ardiles1, Rocio Bruballa1, Martin de Santibañes1, Sebastian Marciano1, Diego Sanchez Thomas1, Francisco J Mattera1,2, Eduardo de Santibañes1, Juan Pekolj1.
1Liver Transplan Unit, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina; 2Liver Transplan Unit, Hospital El Cruce, Florencio Varela, Argentina
Background: Liver retransplantation (re-LT) represents the only treatment for patients with irreversible graft failure. The aim of the current study was to describe the outcomes of both, patient and graft, after re-LT, at a high-volume referral center.
Methods: A retrospective observational cohort of adult patients, with liver disease, who underwent re-LT in our institution between January 1996 and December 2019.
Results: Between 1996 and 2019, 877 liver transplants were performed in adult patients at our Hospital, 49 of which (5.6%) were re-LTs. In 16 cases (32.7%) were early re-LT, while 33 (67.3%) were late. Mean age was 47 (IQR 36-60) and 26 (53%) were male. The main cause of LT was Hepatitis C Virus (HCV) in 17 (35%) patients, while Autoimmune Hepatitis (AIH) was second in frequency, with 9 patients (18.4%). The main indications of early re-LT were arterial thrombosis 9 (56.3%) and primary graft dysfunction 3 (18.8%). For late re-LT, the most frequent causes were primary disease recurrence 15 (45.5%) and biliary stricture 9 (27.3%). Median time to re-LT was 35 months (CI 5-98). 38 patients presented a total of 58 postoperative complications, 21 (36%) of these were immediate, 14 (24%) mediate and 23 (39%) presented late complications. The most frequent complication was bleeding 7 (33%) in the immediate period, rejection 5 (35.7%) in the mediate stage, and primary disease recurrence 10 (43.5%) in the late stage. With a mean follow-up of 3.08 years, there were a total of 13 (26%) deaths, 6 of which were within the first 90 days of surgery and 4 during the first 24 hours. The patient's overall survival (OS) for the first year was 85% (Confidence Intervals (CI) 71-92) and 70% at five years (CI 53-82) Figure 1. Three (6.12%) patients presented loss of graft and were included again in the transplant list; of these, one agreed to a new transplant while the remaining two died. This gave us graft survival results similar to those obtained for the re-LT patient; 85% at one year (CI 71-92) and 70% at 5 years (CI 53-82) Figure 2.
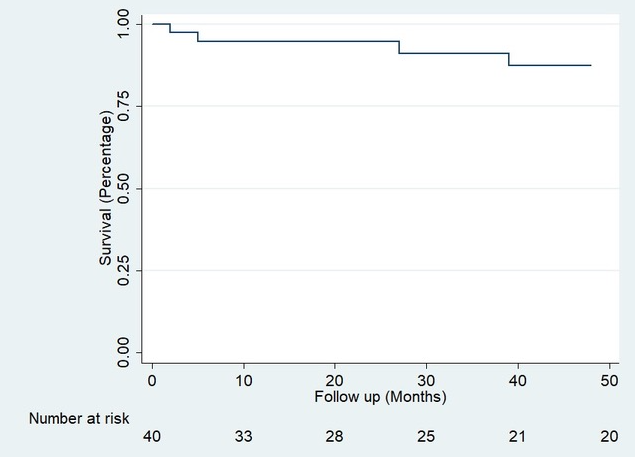
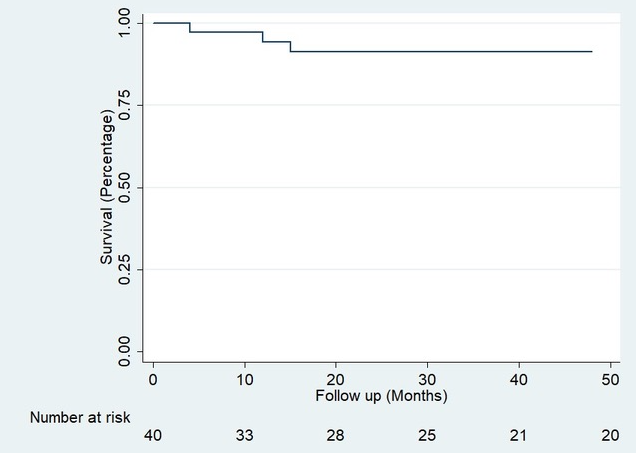
Conclusion: Our study shows that re-LT is a valid and safe treatment for both early graft dysfunction and for transplanted patients who again present end-stage liver disease, showing a satisfactory long-term evolution, with parameters comparable to primary transplantation.
