Humoral response to COVID-19 mRNA vaccines in a cohort of young kidney transplant recipients from a single center in northern Italy
Marco Cazzaniga1, Sara Testa2, Olga Caporale2, Maria Viganoni1, Giovanni Montini2.
1IRCCS Ca' Granda Osp Maggiore Policlinico - Pediatric Nephrology, Dialysis and Transplantation Unit, University of Milan, Milano, Italy; 2Pediatric Nephrology, Dialysis and Transplantation Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy
Objectives: To investigate immune-response to COVID-19 vaccines in young kidney transplant (KT) recipients from Northern Italy.
Methods: We prospectively studied KT patients aged 12-25 years, managed in our Center on maintenance IS therapy (corticosteroids, CNI and anti-proliferative agents), eligible for antiSARS-Cov2 vaccination according to the schedule of the Italian Medicines Agency for immunosuppressed patients (two doses plus additional dose one month later). From 1st July 2021 to 28th February 2022 we evaluated antiSpike-protein antibody response at T0 (before vaccine), T1, T2 and T3 (14±3 days after 2nd and 3rd dose and 90±7 days after 3rd dose, respectively) to BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (Moderna). AntiSpike total Ig titer cut-off was 0.8 U/ml (Roche® Elecsys Anti-SARS-CoV-2-S). Exclusion criteria: KT or additional IS within 6 months, relapse of primary disease, vaccine before KT, ongoing COVID-19, patients resident outside the Region.
Results: Eighty-seven patients were eligible; 68 patients were enrolled. Median age: 19.5 (IQR:16.3-21.9) years; median time from KT: 61.4 (IQR: 36.7-111.7) months. Five patients dropped out of study after enrollment. Anti-SARS-Cov2 Spike Antibodies response to mRNA vaccines is shown in Figure1; 90% of non-responders at T1 (20 patients) seroconverted at T3.
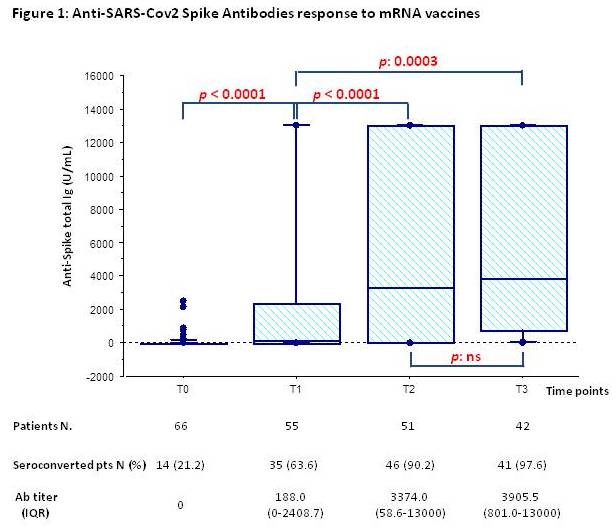
We didn’t find correlation between time from KT (the shorter time, the most intensive immunosuppression) and Ig-titer. Twelve out of 58 pts developed COVID19 after the third additional vaccine dose; in this population AntiSpike Ig titer at T2 was lower compared to the value of non infected patients, even if not statistically significant: 144 U/ml (IQR:9.4-3683) vs. 4771 U/ml (IQR:79.1-13000) respectively. None patient had side effects, including acute rejection episodes or de novo DSA development.
Conclusion: KT pediatric recipients exhibit a satisfactory response after 2 doses of vaccine, that become comparable to that of immunocompetent population after the third. Furthermore, the response after two doses is better if compared with adult KT population (63.6% vs 4-48% ).

right-click to download
