Multicenter study to determine the immune response to the vaccine against SARS COV-2, in patients with chronic kidney disease on dialysis and kidney transplants
Camilo C Ulloa 1, Jaqueline J Pefaur 4,5, Cecilia C Poli6, Emma E Rey-Jurado6, Cecilia C Vial6, Jimena Lina JL Cortés 6, Juan J Hormazábal6, Natalia N González6, Carolina C Ramírez6, Javiera J de la Cruz6.
1Internal Medicine, Clinica Alemana de Santiago, Santiago, Chile; 2Internal Medicine, Clinica Alemana de Santiago, Santiago, Chile; 3Internal Medicine, Universidad del Desarrollo, Santiago, Chile; 4Internal Medicine, Universidad de Chile, Santiago, Chile; 5Centro de trasplante renal, Clínica Santa María, Santiago, Chile; 6Instituto de Ciencias e Innovación en Medicina, Universidad del Desarrollo, Santiago, Chile
Introduction: In Chile, mass vaccination was carried out, prioritizing risk groups for age and comorbidity, applying biannual vaccine boosters. As our knowledge, there are no studies evaluating the humoral and cellular response after the third booster dose in transplantation and dialysis.
Materials and Methods: Multicenter, prospective study in 100 adult patients on hemodialysis (HD) and kidney transplant (KT) recipients were evaluated. Blood samples were taken at the first visit (120 days after the second vaccination - BNT162b2 or SinoVac) and at the second visit (30 days after the 3rd vaccination - BNT162b2). ELISA against IgG Spike SARS-CoV-2 was performed to evaluate humoral response in a total of 47 HD and 53 KT patients. To evaluate cellular response, peripheral mononuclear cells from patients were stimulated with SARS-CoV-2 pool peptides, and IFN-g-secreting cells and SARS-CoV-2-specific T cell response was performed using ELISPOT and flow cytometry, respectively. Cellular immune response was performed in 31 HD and 32 KT patients. Positive cellular immune response was defined as more than 20 spots forming counts (SFC)/million cells and/or percentage of IFN-g+ CD4+ or CD8+ T cells.
Results: After analysis, only statistically significant differences, were found in mean age (in years) of 68.9 ± 13.5 vs 54 ± 12.7 (p<0.0001); kind of work (face-to-face vs homework) 85.1% vs 64.2% (p = 0.02); and in the Charlson Score 6±2.7 (points) vs 4±2.1 when comparing the HD and the KT patients, respectively. The first and second vaccine, was in HD patients mainly Coronavac (88%), while KT patients received BNT162b2 in 69%. The third vaccination, was in all cases with BNT162b2. At first visit, the seroconversion rate was 74% in HD and 48% in KT patients (p=0.001). In KT patients, a seroconversion of 13.4% was observed with Coronavac vs. 64.7% with BNT162b2 (p=0.001) (Figure1).
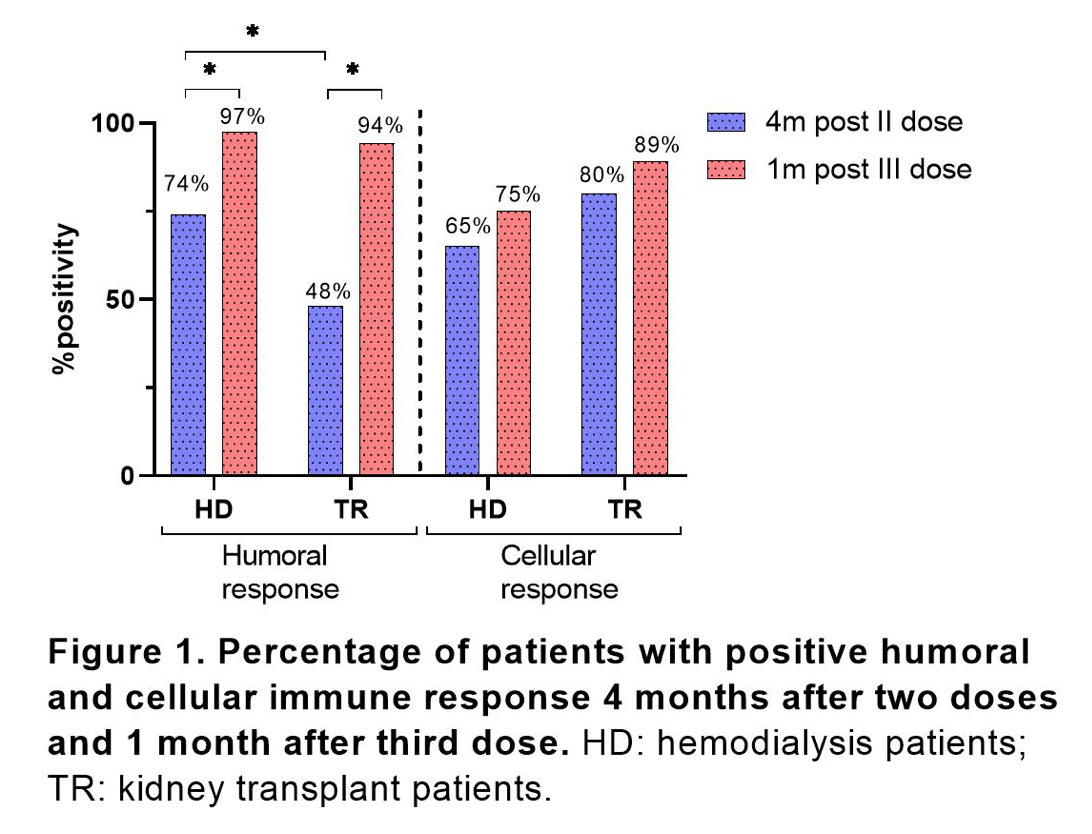
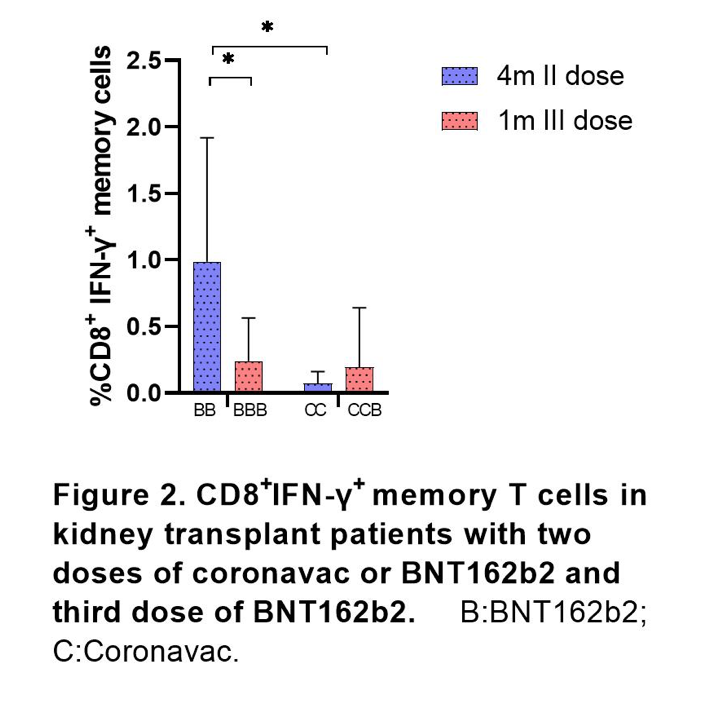
Positive cellular immune response was found in 80% of KT vs 65% of HD patients after second dose. A significant higher CD8+-SARS-CoV-2-specific memory T cells were found in KT patients vaccinated with BNT162b2 as compared to Coronavac (Figure 2). At second visit, a seroconversion was observed in 97.4% HD and 94.3% in KT patients. This increase represented a relative increase of 23% (p=0.02) and 46% in transplant patients (p<0.0001). 89% of kidney transplant while 75% of hemodialysis patients had positive cellular immune response after third dose, showing an increase of 6% and 11% (no significant increase).
Conclusion: Although the humoral response with two doses of the SARS-Cov2 vaccine was poor in transplant patients, especially in those who received the Coronavac vaccine, 83% of patients had cellular immune response. Better cellular and humoral responses with BNT162b2 vaccine were found. There was a significant increase of humoral immune response with the 3rd dose. Altogether, three doses scheme including BNT162b2 are recommended for kidney transplant and hemodialysis patients.
Sociedad de Nefrologia de Chile. Sociedad de Trasplante de Chile. Fundación Pro Salud Renal.

right-click to download
