Antibody response to a third dose of SARS-CoV-2 vaccine in heart and lung transplant recipients
Jennifer Alejo1, Jessica M Ruck1, Teresa PY Chiang1, Allan B Massie4, Aura T Abedon1, Aaron AR Tobian2, Macey L Levan4, Daniel S Warren1, Jacqueline M Garonzik-Wang5, Dorry L Segev4, William A Werbel3.
1Surgery, Johns Hopkins, Baltimore, MD, United States; 2Pathology, Johns Hopkins, Baltimore, MD, United States; 3Medicine, Johns Hopkins, Baltimore, MD, United States; 4Surgery, NYU Langone Health, New York, NY, United States; 5Surgery, University of Wisconsin, Madison, WI, United States
Johns Hopkins COVID-19 Vaccine Study.
Introduction: Heart transplant (HT) and lung transplant (LT) recipients have impaired humoral responses to SARS-CoV-2 vaccination that may be augmented after a third vaccine (D3). It is not known whether HT/LT recipients achieve high levels of anti-spike binding antibody required to neutralize the Omicron variant, and how long this endures after D3.
Methods: A total of 111 HT and 75 LT recipients without prior SARS-CoV-2 infection underwent anti-spike antibody testing with two clinical assays (Roche Elecsys anti-receptor binding domain (RBD) Ig [anti-RBD] and/or Euroimmun Ig [anti-S1] before, 1-, and 3-months after receiving D3 (BNT162b2, mRNA-1273, or Ad26.COV2.S). Population factors and antibody responses were compared using Fisher’s exact testing for categorical variables and Wilcoxon rank-sum testing for continuous variables.
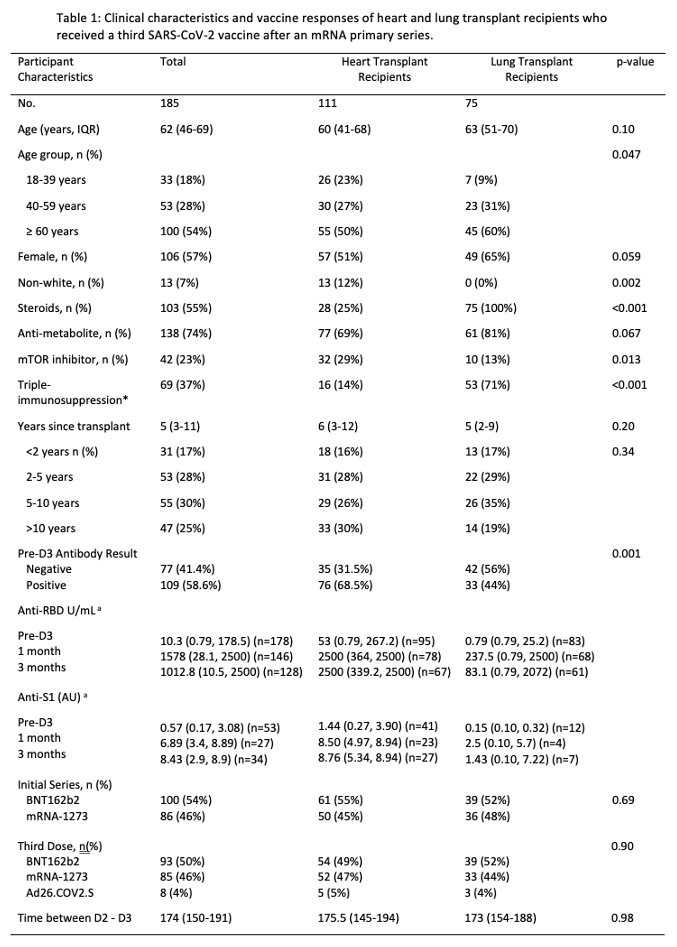
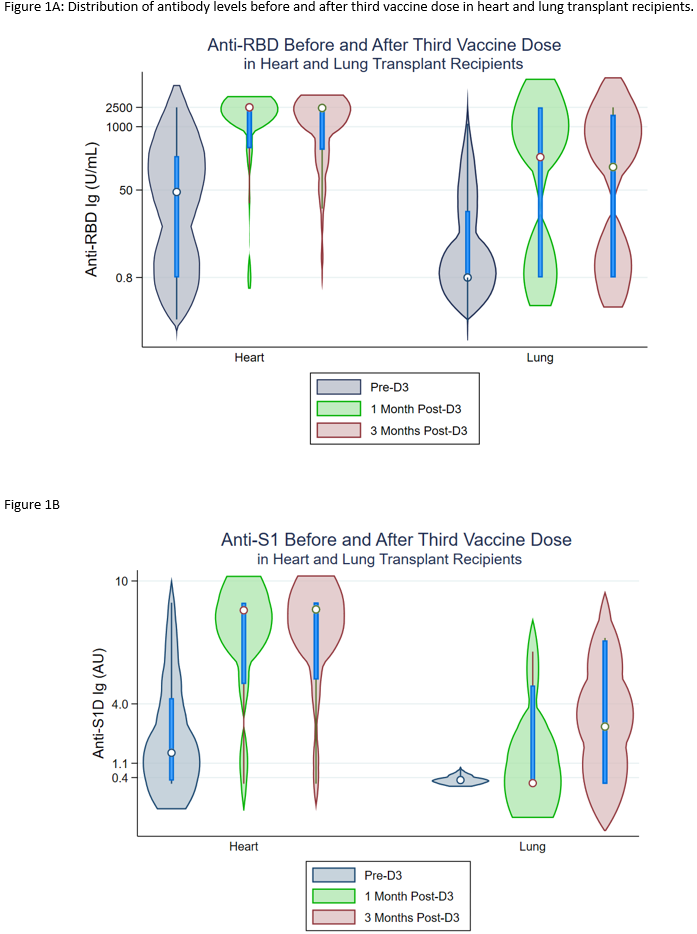
Results: HT and LT recipients were similar with respect to age, sex, and time since transplant (Table). More LT recipients reported triple-immunosuppression (14% vs 71%, p<0.001). Before D3, 35/111(32%) of HT vs 42/75(56%) of LT recipients were negative for anti-spike antibodies. By 1 month post-D3, 24/35(69%) HT and 15/42(36%) LT recipients seroconverted (negative to positive antibody). This was approximately stable at 3 months post D3; 24/35(69%) HT vs 16/39(41%) LT recipients. HT recipients had higher median anti-RBD and anti-S1 antibodies before D3, 1 month, and 3 months post-D3 on both assays at all timepoints (Figure 1A, 1B).
Conclusion: Although many HT recipients develop high-titer antibody response to a third dose of SARS-CoV-2 vaccine, LT recipients demonstrate low seroconversion rates and lower titers, reflecting heavier immunosuppression and suggesting higher COVID-19 risk. Strategies to augment vaccine immunogenicity including additional doses, mixing of platforms, and targeted immunosuppressive reduction may be indicated.
This work was supported by the Ben-Dov family, the Trokhan Patterson family, grants T32DK007713 (Dr. Alejo), K01DK101677 (Dr. Massie), and K23DK115908 (Dr. Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases; grant K24AI144954 (Dr. Segev) from the National Institute of Allergy and Infectious Diseases; and grants U01AI138897 and K23AI157893 (Dr. Werbel).

right-click to download
