Normothermic machine perfusion compared with static cold storage of liver grafts for late liver retransplantation: results of the NAPLES initiative
Angus Hann1,2, Hanns Lembach1, Anisa Nutu1, Nick Murphy1, Mansoor N Bangash1, Desley AH Neil1,2, John L Isaac1, David Bartlett1, John R Isaac1, Neil Rajoriya1, Matthew J Armstrong1,2, Hermien Hartog1,2, M Thamara PR Perera1.
1The Liver Unit, Queen Elizabeth Hospital Birmingham, Birmingham, United Kingdom; 2Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, United Kingdom
Introduction: The pool of donor organs available for patients undergoing liver retransplantation is small, due to the perceived need for an optimal graft. Strategies that can facilitate transplantation of suboptimal organs into retransplant candidates requires investigation. The aim of this study was to determine if patients undergoing liver retransplantation, can be safely transplanted with sub-optimal grafts following normothermic machine perfusion.
Methods: Between November 2018 and November 2021, a prospectively enrolled group of retransplant candidates consented to receive livers with suboptimal features but preserved and viability tested with normothermic machine perfusion (NMP). Donor, graft and clinical outcomes were compared to a retrospective group that underwent retransplantation with a graft preserved via static cold storage (SCS) between January 2015 and November 2021. All recipients received grafts from deceased brain death donors. The primary outcome of this study was 6-month graft and patient survival.
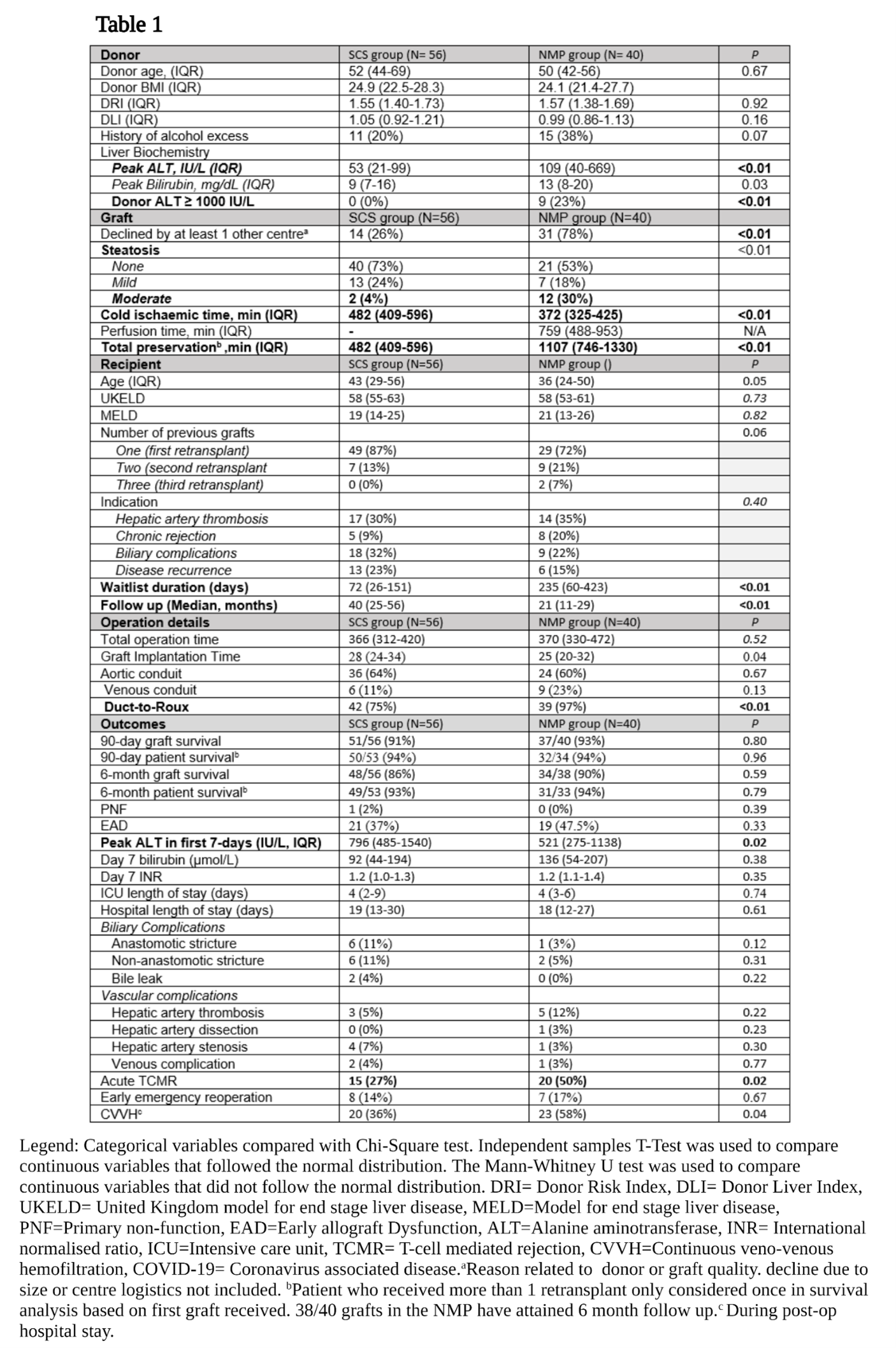
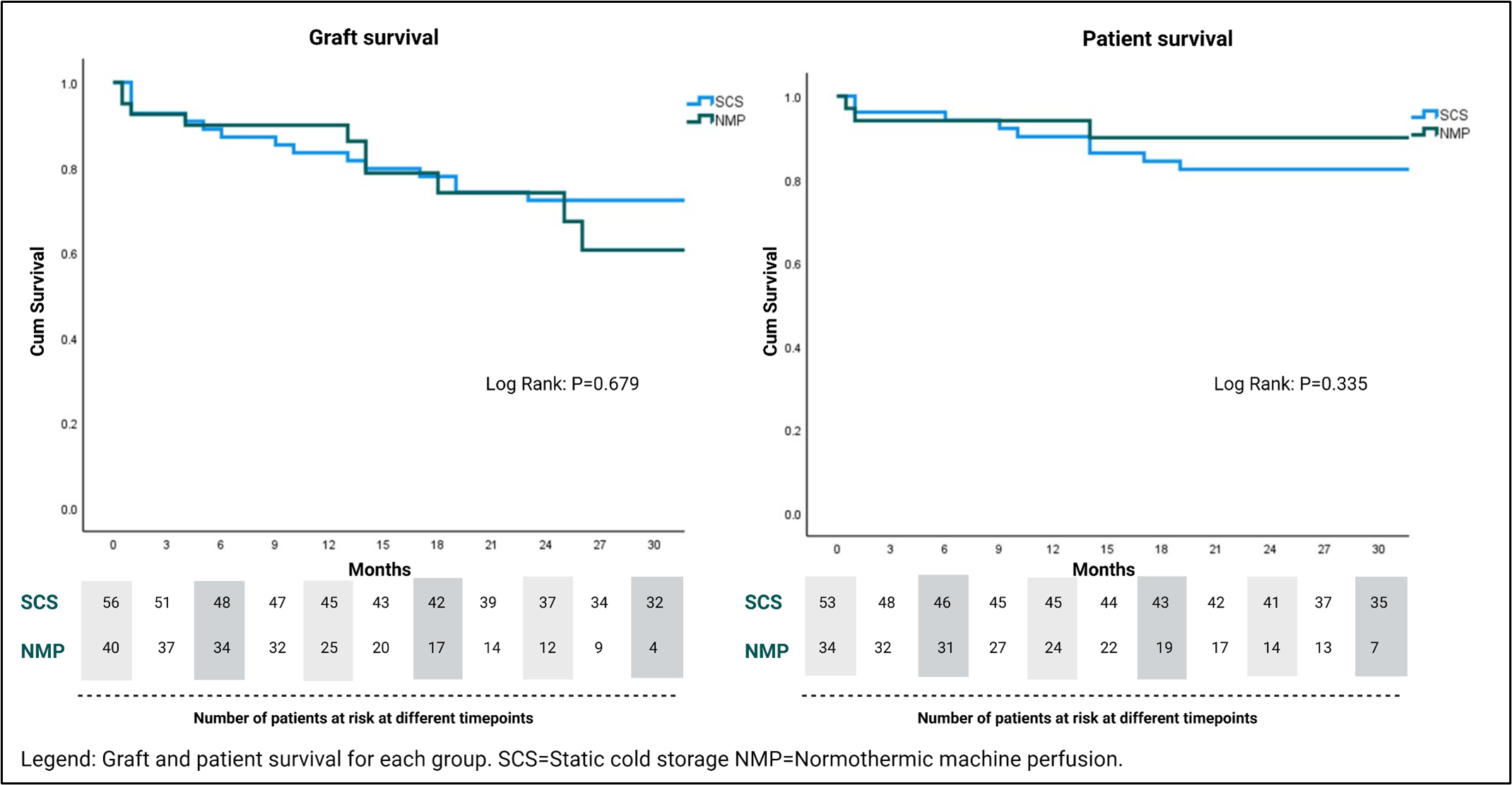
Results: During the study period, 37 patients underwent retransplantation with a NMP preserved graft. Three of these received two NMP preserved grafts. The CS control group comprised 54 patients, and two of these patients received two CS grafts. Therefore, the NMP and CS groups comprised 40 and 56 grafts respectively. Donor, graft, operative and outcome variables are displayed in table 1. The 6-month graft (90% v 86%. P=0.59) and patient (94% vs 93%, P=0.79) survival did not differ between groups (Table 1). This was despite the NMP group having significantly more steatotic grafts (Moderate steatosis; 30% vs 4%, P=<0.01), donors with transaminases >1000 IU/L (0% v 23%, P=<0.01), and grafts previously declined by at least one other transplant centre (78% v 26%, P=<0.01) (Table 1). The peak alanine transaminase in the initial post-operative 7 days was significantly lower (521 vs 796, P=0.02) in the NMP group, however the rate of early acute rejection was higher (50% vs 27%, P=0.02) but did not impact graft survival. Significantly higher rates of CVVH in the NMP group did not translate to a longer length of stay in ICU. Furthermore, longer term graft and patient survival did not differ between groups (Figure 1).
Conclusion: This is the first study to demonstrate that NMP technology can be safely used for sub-optimal liver grafts, and these can be safely transplanted into patients that require retransplantation. This technology can therefore expand the graft options available to this high-risk group.
Anne Fox Foundation, University Hospitals Birmingham Charity.

right-click to download
