
Extracellular vesicles as mediators of infectious tolerance in human alloreactive regulatory cells
Brittany Schreiber1, Sudipta Tripathi1, Paloma Martin-Moreno1, Rohan Saranu1, George Kavalam1, Ana Maria Waaga-Gasser1, Anil Chandraker1.
1Nephrology, Brigham and Women's Hospital, Boston, MA, United States
Introduction: We have developed a method for ex vivo expansion of donor antigen specific T regulatory cell enriched lines (ASTRLs) from stable human kidney transplant recipients (KTRs). Rat derived ASTRLs induced prolonged graft survival and donor specific tolerance in a rat kidney transplant model. Human ASTRLs are suppressive in their phenotype and function. We examined the various mechanisms of ASTRL mediated suppression including extracellular vesicle (EV)-mediated suppression.
Method: ASTRLs were expanded ex vivo from PBMCs of stable KTRs by stimulation with donor allopeptides in presence of IL-2. ASTRLs were characterized for conventional Treg markers by flow cytometry. Functional characterization of ASTRLs were performed using standard suppression assays. Extracellular ATP hydrolysis was determined by malachite green assay. EVs were isolated from the ASTRL expansion media via differential ultracentrifugation and characterized by liquid chromatography-mass spectrometry.
Results: The suppressive mechanism of ASTRLs was alloantigen-specific and dependent on cell-cell contact as shown in Figure 1 (top panel). ASTRLs upregulated the expression CD39 and CD73, ectonucleotidases associated with the adenosinergic pathway, and ASTRLs demonstrated increased extracellular ATP hydrolysis (eATP) that was inhibited by POM1, a CD39 specific inhibitor (Figure1 left bottom panel) which also abrogated the suppressive effect of ASTRLs. In KTRs ASTRLs specific for one donor alloantigen were able to suppress an effector response against other donor alloantigens demonstrating bystander/ linked suppression and inhibit effector T cell proliferation in response to both direct and indirect allorecognition (Figure 1 bottom right panel).
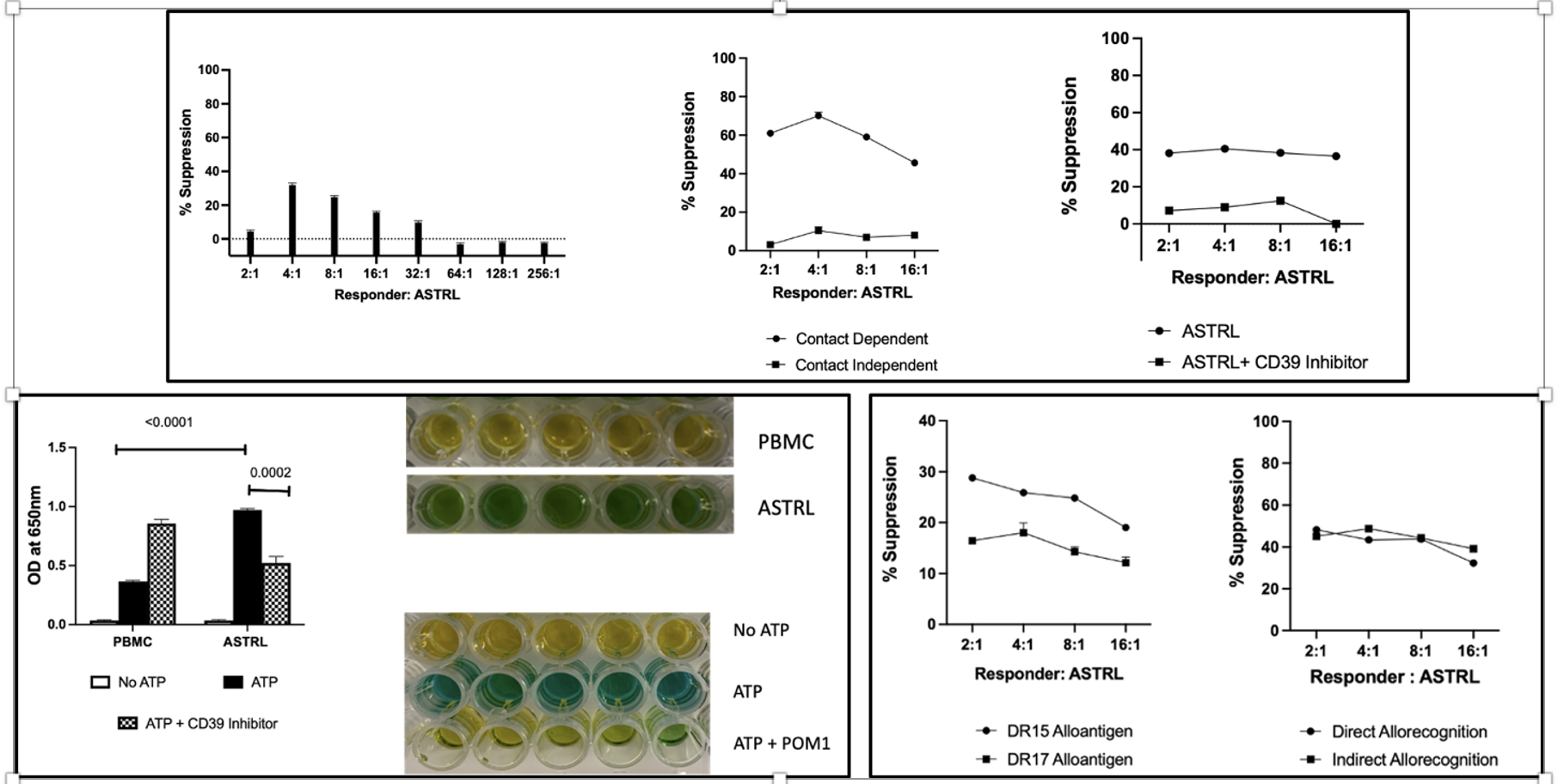
EVs isolated from ASTRLs enhanced the suppressive ability of ASTRLs and hydrolyze eATP independently, which was inhibited by POM1 (Fig. 2) and increase production of IL-4, IL-13, and IL-1RA by alloantigen stimulated T cells, indicating a biasing towards a regulatory response (Fig. 2). Global proteomics identified 1,709 unique proteins in the large EV fraction and 1,356 unique proteins in the small EV fraction. Pathway analysis revealed that both EV fractions under stimulation were enriched for proteins involved in T cell receptor (TCR) signaling and Treg differentiation (Fig. 2) with 145 differentially enriched proteins (DEPs) in the large and 162 DEPs in the small EVs (q<0.05). Pathway analysis of DEPs will be presented.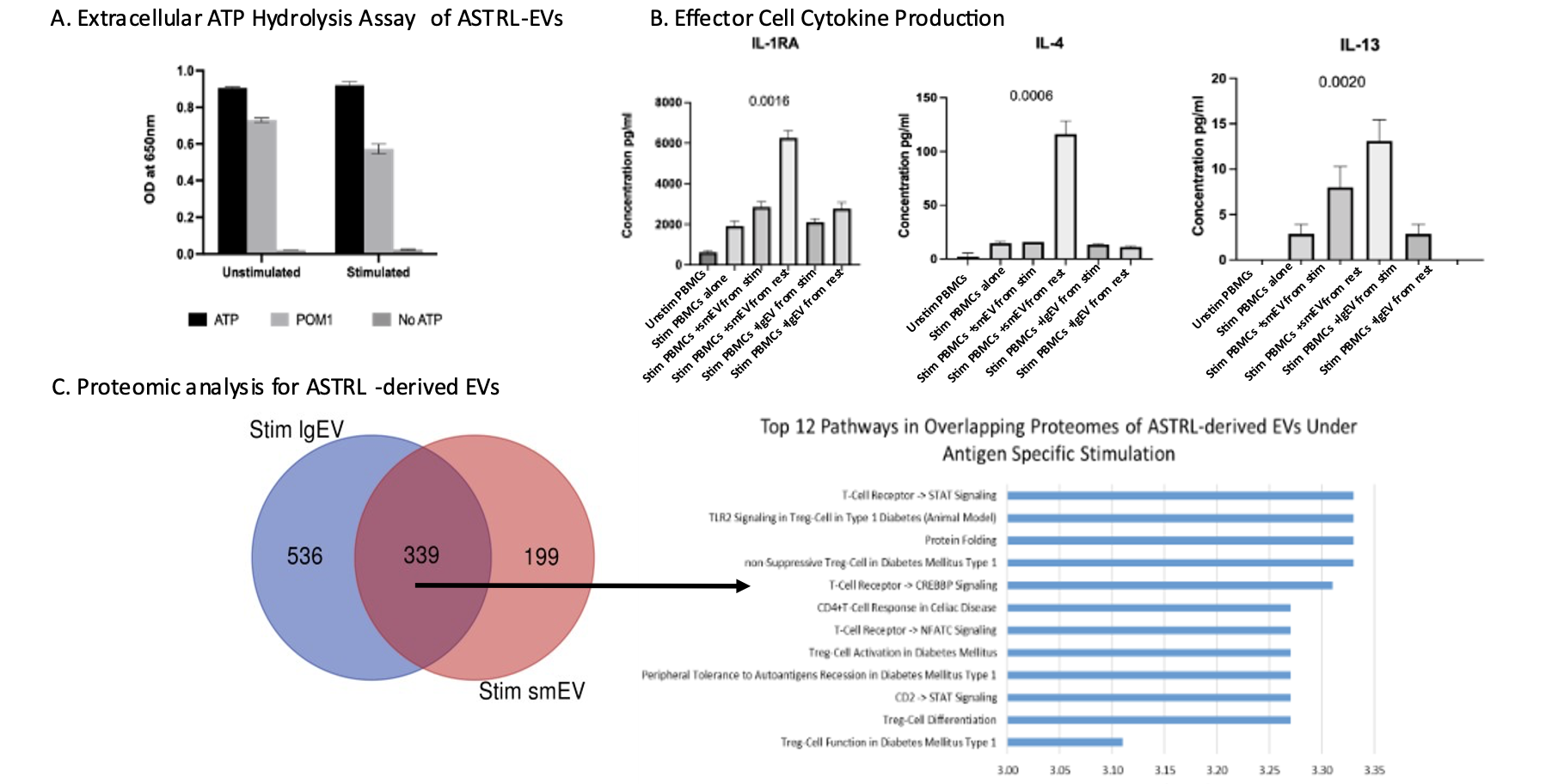
Conclusion: ASTRLs expanded from stable KTRs suppress donor antigen specific effector responses predominantly via the adenosinergic pathway and demonstrate bystander/linked immunosuppression. ASTRL-EVs enhance the suppressive capacity of ASTRLs and exhibit an increase in CD39 function. These findings indicate that human ASTRLs may be useful as autologous regulatory cell therapy in transplant recipients and that ASTRL related EVs may be useful adjuncts to ASTRL cell therapy.
Saxena Transplantation Fund.

right-click to download
