A gelatin hydrogel nonwoven fabric improves outcomes of subcutaneous islet transplantation
Norifumi Kanai1, Akiko Inagaki2, Yasuhiro Nakamura3, Takehiro Imura2, Hiroaki Mitsugashira1, Ryusuke Saito1, Shigehito Miyagi1, Takashi Kamei1, Michiaki Unno1, Yasuhiko Tabata4, Masafumi Goto1,2.
1Department of Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Division of Transplantation and Regenerative Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan; 3Division of Pathology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Sendai, Japan; 4Laboratory of Biomaterials, Department of Regeneration Science and Engineering, Institute for Frontier Life and Medical Sciences, Kyoto University, Kyoto, Japan
Introduction: Subcutaneous islet transplantation is a promising treatment for severe diabetes, since it has several advantages, including minimal invasiveness and easy accessibility for the islet grafts, which makes it possible to monitor and/or remove islet grafts if needed. However, poor vascularization has long been regarded as major drawback in this transplant site. Recently, several groups have reported that the compensation of extracellular matrices (ECM) is also crucial for successful subcutaneous transplantation. We previously reported that a recombinant peptide (RCP) enhances subcutaneous islet engraftment. However, it is impractical for clinical use because RCP must be removed when transplanting islets. We herein investigated whether a novel bioabsorbable gelatin hydrogel nonwoven fabric (GHNF) could improve subcutaneous islet engraftment.
Methods: A silicon spacer with or without GHNF was implanted into the subcutaneous space of diabetic mice at 6 weeks before islet transplantation. Syngeneic islets (400 islet equivalents) were transplanted into the pretreated space or intraportally (Ipo group). Blood glucose, intraperitoneal glucose tolerance, immunohistochemistry, CT angiography and gene expression were evaluated.
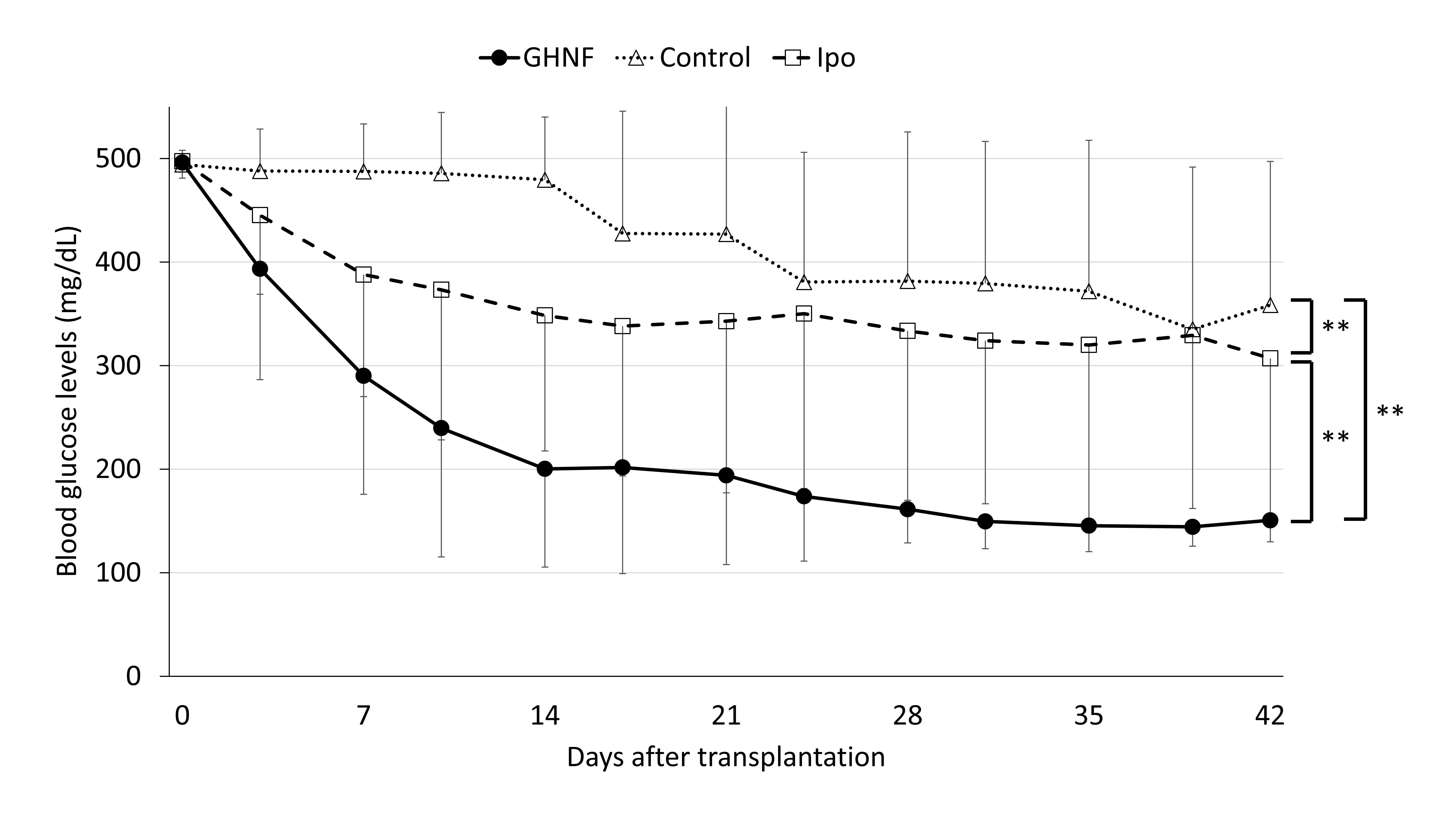
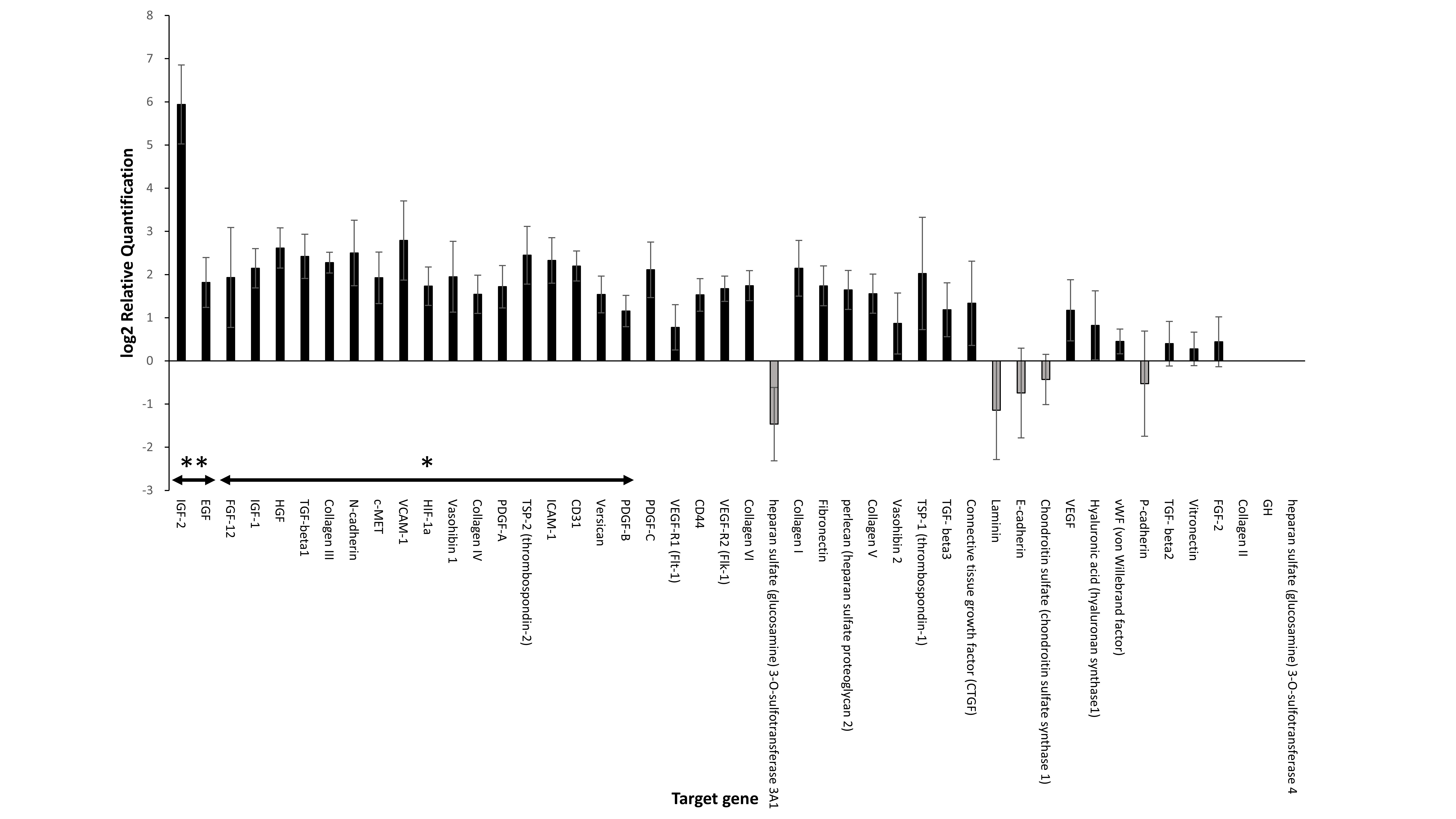
Results: The cure rate (Figure 1) and glucose tolerance of the GHNF group were significantly better than in the control and Ipo groups (p<0.01, p<0.05, respectively). In the GHNF group, a limited increase of vWF-positive vessels was detected in the islet capsule, whereas laminin (p<0.05), collagen III and IV were considerably enhanced. CT angiography revealed that the blood vessel volume surrounding the silicon spacer was comparable between the GHNF and control groups. TaqMan arrays revealed a significant upregulation of 19 target genes (including insulin-like growth factor-2(IGF-2)) in the pretreated space (p<0.05) (Figure 2). The protein level of IGF-2 in the supernatant of the homogenized subcutaneous fibrous capsules in the GHNF group was also significantly higher than that in the control group.
Conclusions: Pre-implantation of a GHNF effectively improved subcutaneous islet engraftment, resulting in much better outcomes in comparison to intraportal islet transplantation. This beneficial effect may be mainly due to the compensation of ECM for the islet capsule and protection of islet viability by various growth factors, rather than the enhancement of neovascularization.
This study was supported by a Japanese Grant-in-Aid for Scientific Research (A) (Grant Number 18H04056) from the Japan Society for the Promotion of Science. This study was supported by AMED under Grant Number JP19bm0404043. The authors thank Kozue Imura and Megumi Goto (Division of Transplantation and Regenerative Medicine, Tohoku University) for their excellent technical assistance. The authors also acknowledge the support of the Biomedical Research Core of Tohoku University, Graduate School of Medicine and TAMRIC (Tohoku Advanced Medical Research and Incubation Center).

right-click to download
