The screening and treatment of asymptomatic bacteriuria do not reduce the incidence of urinary tract infection or pyelonephritis in the first two months after renal transplantation: a randomized controlled trial
Jose Manuel Arreola Guerra1,3, Alfredo Chew Wong1,3, Emmanuel Fuentes Hernandez1, Emmanuel Antonio Mendoza Enciso3, Mario Gonzalez Gamez3, Margarita Guadalupe Ortiz Lopez1, Dante Rodriguez Jimenez1, Teresa Tiscareño Gutierrez1, Ana Lilia Reza Escalera1, Enrique Gil Guzman2, Luis Romo Franco1,2, Rafael Reyes Acevedo1,2.
1Nephrology, Centenario Hospital Miguel Hidalgo, Aguascalientes, , Mexico; 2Kidney Transplant, Centenario Hospital Miguel Hidalgo, Aguascalientes, , Mexico; 3Internal Medicine, Centenario Hospital Miguel Hidalgo, Aguascalientes, , Mexico
Background: The incidence of asymptomatic bacteriuria (AB) in the first two months after renal transplantation (pRT) is very high. Due to increased immunosuppression and the use of urinary devices, the screening and treatment of AB in this period could reduce the frequency of urinary tract infection (UTI) and pyelonephritis.
Objective: To evaluate the efficacy of AB screening and treatment in reducing the incidence of UTI in the first two months after kidney transplantation.
Methodology: Randomized, single-blind, controlled clinical trial of pRT patients. Urine culture was conducted on the day of bladder catheter removal, week three pRT, and prior to removal of the ureteral catheter. Patients assigned to the intervention group received treatment for AB based on an antibiogram for 5 days. The control group did not receive treatment. The primary outcome was incidence and time to the first episode of UTI and graft pyelonephritis.
Results: 80 patients were included, 40 randomized to each group. The average age was 29.8 years and 33.7% were women. The cause of chronic kidney disease was classified as "unknown" in 88.7% and only 2 patients had Diabetic Nephropathy (1.25%). The source of the kidney donation was mostly from a living donor (86.2%). The frequency of AB in the intervention group was lower than in the control group (17.5 vs 37.5%, p=0.04). The incidence and time to first UTI and pyelonephritis were higher in the intervention group (25 vs 10%, p=0.07 and 15 vs 2.5%, p= 0.04) (Figure 1). Also, the number of UTI was higher in the intervention group (5 vs 18, p= 0.05) and the frequency of recurrent UTI (17.5 vs 2.5%, p =0.05). The most common isolated bacteria was E. Coli (n= 28, 59.5%) and more than half were E. Coli ESBL (n=15). Bacterial resistance did not differ between intervention groups.
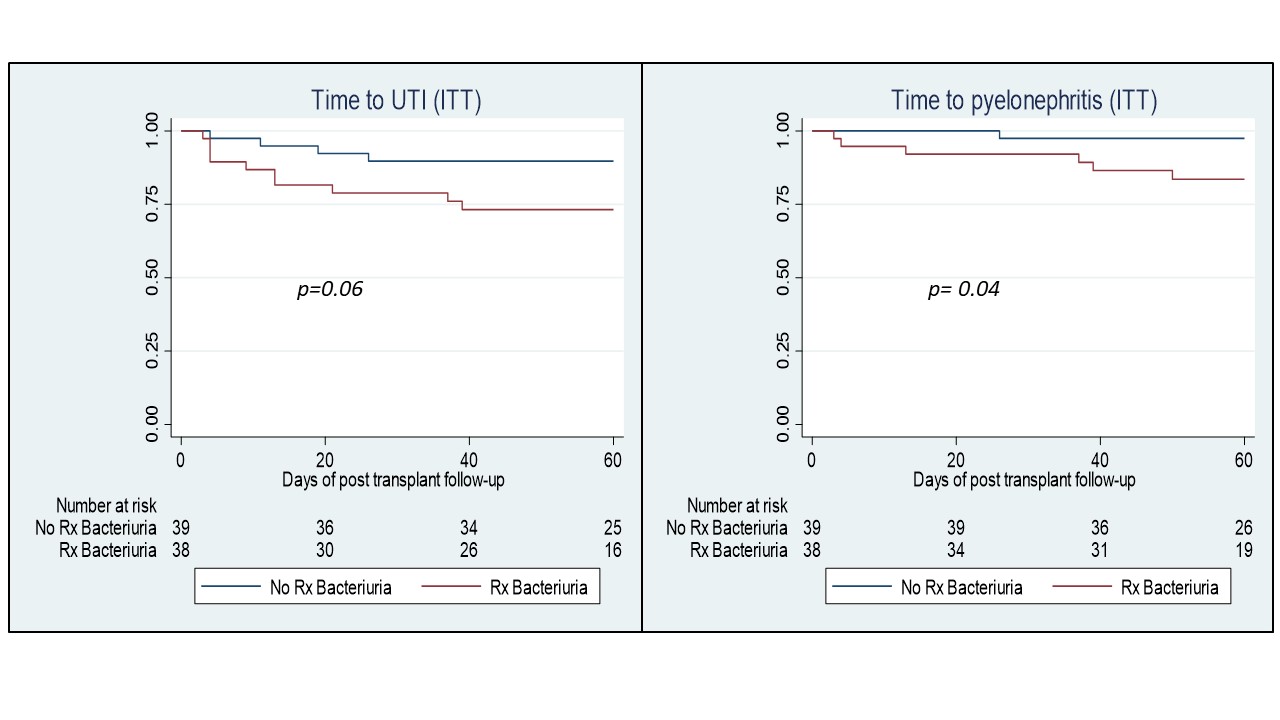
Conclusions: The screening and treatment of AB in the first two months pRT does not reduce the incidence of UTI or graft pyelonephritis and probably could increase their frequency. Generalized treatment of AB during the first months after renal transplantation should be avoided. (Clinical Trials identifier: NCT04333602)
Astra Zeneca and AMGEN.

right-click to download
