Real-world treatment patterns and outcomes for cytomegalovirus infection and disease in hematopoietic stem cell transplant recipients in selected countries outside of North America and Europe: a systematic review
Sung-Yeon Cho1, Depei Wu2, Clarisse M Machado3, Inderjeet Singh4, Anudeep Sandhu5, Dirk Demuth5, Monica Slavin6.
1Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea; 2First Affiliated Hospital of Soochow University, Suzhou, People's Republic of China; 3Institute of Tropical Medicine, University of São Paulo (IMT-FMUSP), São Paulo, Brazil; 4Takeda Pharmaceuticals India Pvt Ltd, Gurgaon, India; 5Takeda International AG – Singapore Branch, Singapore, Singapore; 6Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne, Australia
Background: There is a paucity of real-world data on treatments used for cytomegalovirus (CMV) in hematopoietic stem cell transplant (HSCT) recipients, which differ across countries, outside of developed markets. To address this knowledge gap, a systematic review was conducted to evaluate current treatment patterns for CMV infection and disease following HSCT in selected countries outside of North America and Europe and describe related outcomes.
Methods: Information sources (search period: 01 Jan. 2011 – 21 Jul. 2021) included indexed literature (Ovid® MEDLINE and Embase, Cochrane Database of Systematic Reviews and World Health Organization database Global Index Medicus), conference abstracts, pragmatic searches of the grey literature and snowballing of references lists of retained studies. Observational studies of interest included HSCT recipients (any age) who developed CMV infection or disease in 15 selected countries in Asia-Pacific, Latin America, Russia, and the Middle East. Outcomes of interest were treatment patterns for CMV infection and CMV disease, proportion of patients (pts) with resistant and refractory CMV including definitions, treatment-related outcomes and adverse events (AEs). The protocol was registered in PROSPERO (CRD42020205559).
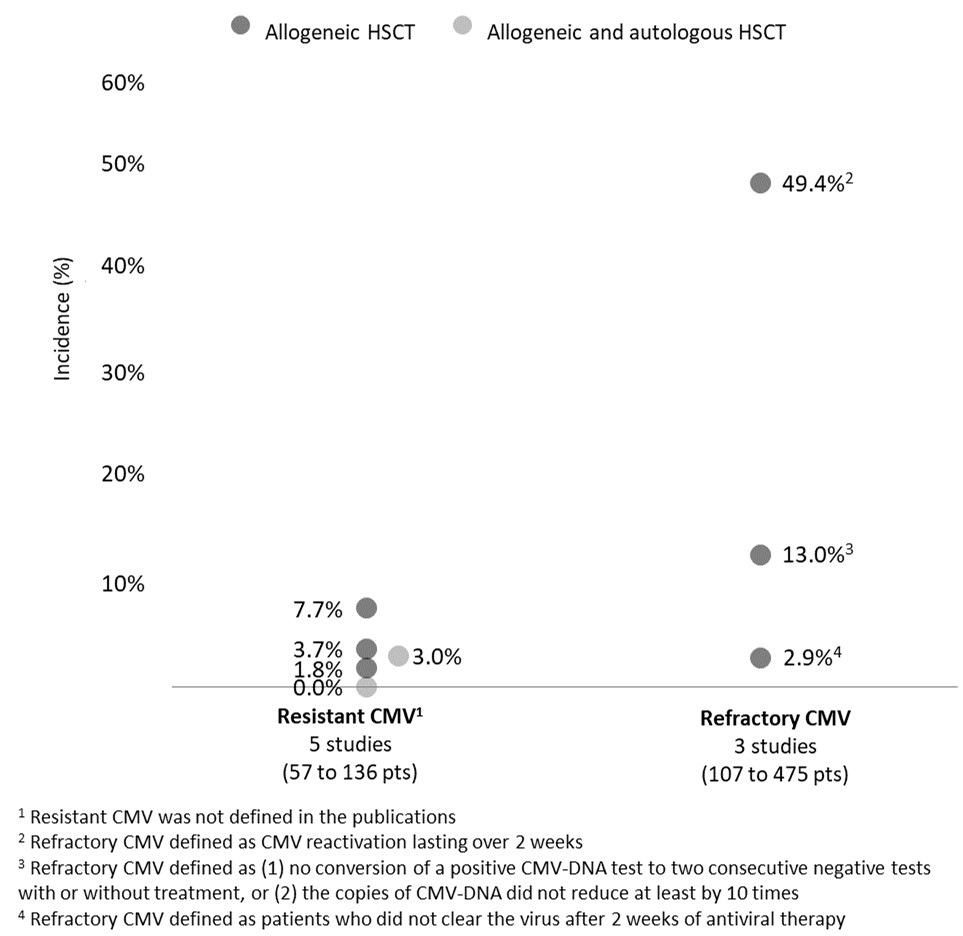
Results: Out of 25 retained studies (33 to 475 pts), most included allogeneic HSCT recipients (n=21, 84.0%) and adult pts (n=20, 80.0%). In HSCT recipients, pre-emptive therapy with intravenous ganciclovir (IV GCV) and/or valganciclovir (VGCV) is the conventional first-line (1L) approach for CMV infection prevention, with a median treatment duration of 14-24 days (5 studies). GCV IV was also the conventional treatment for CMV disease (5 studies), with treatment lasting 12-30 days (3 studies). Neutropenia was observed in 30.0% and 39.8% pts treated with GCV and, in 3 studies, neutropenia, myelosuppression, and nephrotoxicity led to GCV discontinuation, respectively, in 13.6%, 10.0%, and 2.3% of pts. In 3 studies, up to 10.0% of pts had second-line (2L) treatment with foscarnet, due to GCV-related myelosuppression. The reported proportion of pts with resistant CMV (definition not specified) ranged between 0% and 7.7% (5 studies; Figure 1). These pts received foscarnet or cidofovir as 2L therapy for a mean duration of 14 days (no data on AEs reported). In 3 studies, estimates of refractory CMV were 2.9%, 13.0%, and 49.4% (latter estimate reported in 39 pts, of whom 56.0% had recurrent CMV).
Conclusion: In selected countries outside of North America and Europe, conventional therapeutic options for pre-emptive and treatment of CMV infection and CMV disease following HSCT were IV GCV and VGCV. However, premature treatment discontinuation occurred in up to 1 in 8 pts, highlighting an unmet need with current standard of care. Real-world outcomes in pts with refractory CMV (with or without resistance) were scarce and warrants further investigation in this patient population, including therapeutic management.
This review was funded by Takeda International AG – Singapore Branch. S-YC has served as a consultant for Takeda Pharmaceutical, and has received research support and payment for lectures from Merck Sharp & Dohme, Pfizer and Gilead. WD has no conflicts of interest to disclose. CMM has received honoraria from Takeda and MSD (advisory board, speaker, educational events, meetings). IS is an employee of Takeda Pharmaceuticals India Pvt Ltd. AS and DD are employees of Takeda International AG – Singapore Branch and hold stock options. MS has received grants from F2G, Gilead, Merck and personal fees from Gilead, Pfizer, Takeda and Roche for work outside of this research. Literature retrieval, analysis and medical writing support were provided by Aurore Bergamasco, Camille Goyer, and Yola Moride of Yolarx Consultants and funded by Takeda International AG – Singapore Branch.

right-click to download
