Equal outcome and fewer complications with azathioprine compared to mycophenolate in pediatric first-time kidney transplantation: should current practice be re-evaluated?
Felicia Kjaernet1,2, Mia Herthelius3, Helena Genberg1,2.
1Dept of Transplantation Surgery, Karolinska University Hospital, Stockholm, Sweden; 2Div of Transplant Surgery, CLINTEC, Karolinska Institutet, Stockholm, Sweden; 3Dept of Pediatric Nephrology, Karolinska University Hospital, Stockholm, Sweden
Background: In pediatric kidney transplantation, the immunosuppressive standard practice has shifted over the past two decades. Mycophenolate has largely come to replace azathioprine as the antimetabolite of choice worldwide. The evidence in support of mycophenolate with regards to graft survival in adult kidney transplantation is solid. Yet, there are few studies comparing azathioprine and mycophenolate in pediatric kidney transplantation. The aim of this study was therefore to compare outcome and early complications, using a immunosuppressive protocol including azathioprine or mycophenolate in combination with tacrolimus and prednisolone ± basiliximab induction in pediatric kidney transplantation.
Methods: From 2003 to 2018, 106 pediatric patients (≤16 years) underwent kidney transplantation at Karolinska University Hospital. Patients undergoing re-transplantation or ABO-incompatible transplantation were excluded. In total, 84 first-time kidney recipients receiving standard immunosuppression with tacrolimus and prednisolone ±basiliximab, combined with either mycophenolate or azathioprine were included. Antimetabolite on the day of surgery was used as grouping label in the analysis: 38 patients (45%) received azathioprine (start dose 3 mg/kg), with 0% basiliximab induction (AZA group) and 46 patients (55%) received mycophenolate mofetil (start dose 600 mg/BSA), 11% with basiliximab induction (MMF group).
Results: Mean follow-up (10.4 ± 5.1 yrs) and mean age (7.9 ± 4.9 yrs) did not differ between groups. There was however a difference in dialysis dependence (74% in the MMF group vs 50% in the AZA group, p=0.02) and donor source (67% living donor recipients in the MMF group and 92% in the AZA group, p=0.006). In the MMF group 9 patients were PRA+, but only one patient had PRA > 10%. Patient survival and graft survival were similar across groups (overall 95.1% and 83.5% at 10 yrs). Measured GFR was at 5 yrs similar in the two groups: 51.1±30.6 ml/min/1.73 in the MMF group and 51.3±23.2 ml/min/1.73 in the AZA group (p=0.98). More unscheduled readmissions were observed in the MMF group first year (70% vs 47%, p=0.04), primarily owing to urosepsis (18% vs 0%, p=0.02) and diarrhea (24% vs 6%, p=0.04), No difference was observed between groups regarding rejection episodes (overall 18%) and transplant related infections (CMV/EBV/BKV) during the first year after transplantation.
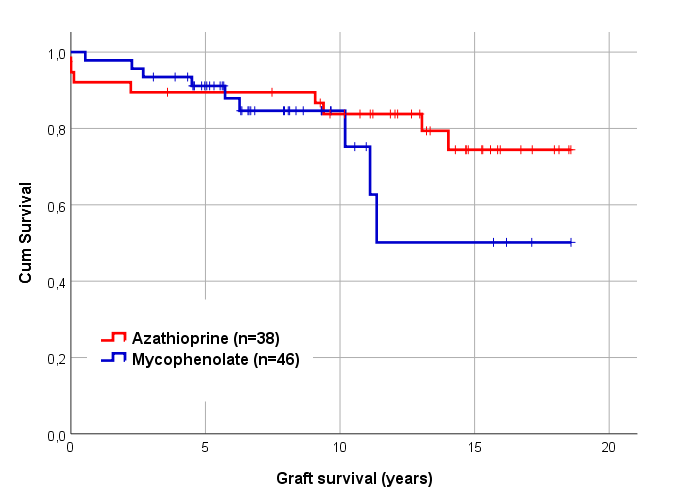
Conclusion: Based on the experience in adult kidney transplantation, mycophenolate has become the preferred antimetabolite in pediatric kidney transplantation. However, our data suggest that azathioprine is not inferior to mycophenolate in immunologic low-risk pediatric kidney recipients with similar graft survival and potentially fewer complications. However, giving the limitations of this single-center study, further investigations with larger study populations are needed.
Swedish Order of Freemasons.

right-click to download
