ABO-incompatible liver transplantation is a safe and effective intervention for paediatric recipients
Ashwin Bhana1, Gordon Thomas2, Albert Shun2, Susan Siew1, Edward O'Loughlin1, Janine Sawyer1, Brooke Andersen1, Michael O Stormon1.
1Gastroenterology, Children's Hospital at Westmead, Sydney, Australia; 2Surgery, Children's Hospital at Westmead, Sydney, Australia
Introduction: ABO-incompatible (ABOi) Liver transplants in adults are performed infrequently, usually for urgent indications when ABO compatible grafts are unavailable and often require additional desensitisation therapy with mixed outcomes. Several studies have demonstrated comparable rates of graft survival in children less than 2 years of age who receive ABOi Liver transplants for non-urgent indications compared to ABO-compatible (ABOc) recipients without the need for desensitisation therapy. These studies have reported mixed outcomes with regards to rejection, biliary complications and rejection. We compared outcomes of children who received an ABOi graft with those who received an ABO compatible (ABOc) graft in a single paediatric transplant centre.
Method: A retrospective review of patients aged 0-16 years inclusive who had received ABOi Liver transplants from 1986 to 2020 at the Children’s Hospital at Westmead, the Paediatric arm of the Australian National Liver Transplant Unit, Sydney, Australia. Comparison of post-transplant outcomes between first-time ABOi and ABOc recipients was made between 2008-2020.
Results: 423 paediatric liver transplants were performed, of which 30 were ABOi. Between 1989 and 1997 6 out of 85 (7.1%) ABOi transplants occurred, at a median age of 7.18. No further ABOi transplants occurred from 1998 to 2007. From 2008 to end of 2020 there were 24 ABOi grafts in children at median age of 0.77. Standard immunosuppression for both groups included tacrolimus (or cyclosporin prior to 1998) and corticosteroids. Basiliximab (interleukin-2 receptor antibody) or a combination of Basiliximab and Rituximab (anti-CD20 antibody) was used more commonly in the ABOi group (63% and 17% respectively) than the ABOc group (29% and 0.6% respectively). ABOi recipients who received Rituximab were older with a median age of 6.22 (range 4.47-9.01 years) compared to those who didn’t receive Rituximab (median age 0.71, range 0.48-3.64). There was no statistically significant difference in rates of acute cellular rejection (42% vs 34%), antibody mediated rejection (8.2% v 2.4%), sepsis (42% vs 29%), biliary strictures (13% vs 12%) or thrombotic events (13% vs 8.4%) between ABOi and ABOc groups. Graft survival at 5 years post-transplant was 95.8% for ABOi patients and 86.1% for ABOc patients.
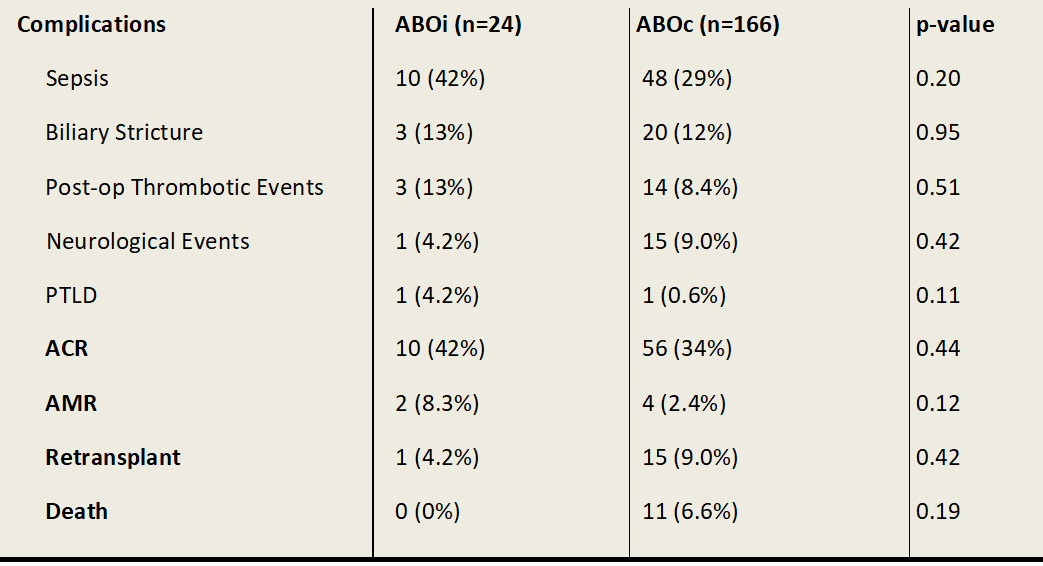
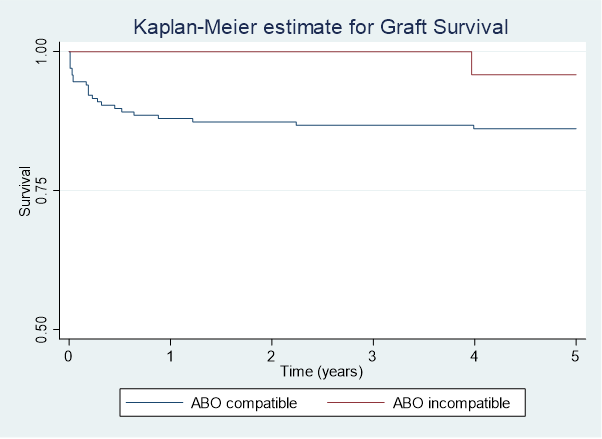
Conclusion: ABOi liver transplantation in paediatric patients is a safe and successful intervention when undertaken in the appropriate setting. Our centre has demonstrated comparable complication rates between ABOi and ABOc recipients. The role of conditioning therapy with Basiliximab or Rituximab is uncertain in the young ABOi liver transplant recipients.

right-click to download
