Single-dose rituximab induction may prevent EBV viremia in paediatric kidney recipients long-term: an investigation from the nordic paediatric renal transplantation study group
Helena Genberg1,2, Felicia Kjaernet1,2, Anna Bjerre3,4, Zivile Bekassy5, Amir Sedigh9, Helle Thiesson6, Hjördis Thorsteinsdottir3, Anna Varberg Reisaeter8, Susanne Westphal7, Anders Åsberg8.
1Dept of Transplantation Surgery, Karolinska University Hospital, Stockholm, Sweden; 2Div of Transplant Surgery, CLINTEC, Karolinska Institutet, Stockholm, Sweden; 3Division of Paediatric and Adolescent Medicine, Olso University Hospital, Oslo, Norway; 4Institute of Clinical Medicine, University of Oslo, Oslo, Norway; 5Dept of Pediatric Nephrology, Lund University, Lund, Sweden; 6Dept of Nephrology, University of Southern Denmark, Odense, Denmark; 7Dept of Pediatrics, University of Gothenburg, Gothenburg, Sweden; 8Dept of Transplantation Medicine, Olso University Hospital, Oslo, Norway; 9Dept of Surgical Sciences, Transplantation Surgery, Uppsala University, Uppsala, Sweden
Nordic Paediatric Renal Transplantation Study Group (NPRTSG).
Background: In 2003 we introduced the B-cell depleting agent rituximab as induction therapy for paediatric immunologic high-risk kidney transplantations (KT). Still, the use of rituximab induction has been limited and primarily restricted to ABO-incompatible KT. In adults, rituximab induction has been evaluated in two placebo-controlled trials as prophylactic anti-rejection therapy in KT (Tydén 2009 & van Hoogen 2015) However, although rituximab appeared safe, none of these studies could prove any clear benefit of the agent within 180 days of follow-up. In paediatric KT no such prospective trial has been performed. In fact, little has been reported on rituximab induction in paediatric organ transplantation. With the aim of evaluating long-term effects of rituximab induction, we conducted a retrospective multi-centre study within the Nordic Paediatric Renal Transplantation Study Group (NPRTSG), comparing paediatric KT with and without rituximab induction.
Methods and Materials: Relevant data was retrieved from the Scandiatransplant registry, the NPRTSG registry and the electronic medical records. Six out of 11 NPRTSG centres had used rituximab induction in paediatric kidney transplantation (recipient age < 16 years) during the study period (2003-2018). A complete set of data was available for 240 paediatric first-time kidney recipients and further analysed, 27 (11%) with single-dose rituximab induction (RIT group) and 213 (89%) without rituximab induction (NoRIT group). A thorough analysis of outcome long-term including infections and other complications was undertaken.
Results: Follow-up was 9 years (± 4.4) overall. Patient survival in the RIT group was 100% at 10 years compared with 96.8% in the NoRIT group (p=ns) and graft survival 92.9% in RIT group compared with 84.6% in the NoRIT group (p=ns). In the RIT group 30.8% of patients were treated for acute rejection first year vs. 15.6% in the NoRIT group (p=0.09). EBV serologic mismatch status did not differ significantly between groups (41% overall). EBV mismatch was however associated with EBV viremia in the NoRIT group (OR 4.1) (p<0.001). No patient (0%) in the RIT group was diagnosed with EBV viremia within 36 months of KT. The incidence of CMV and BK viremia was similar in the two groups. Seven patients (3.3%) in the NoRIT group were diagnosed with malignancy during follow-up (5 with PTLD) compared with no one in the RIT group.
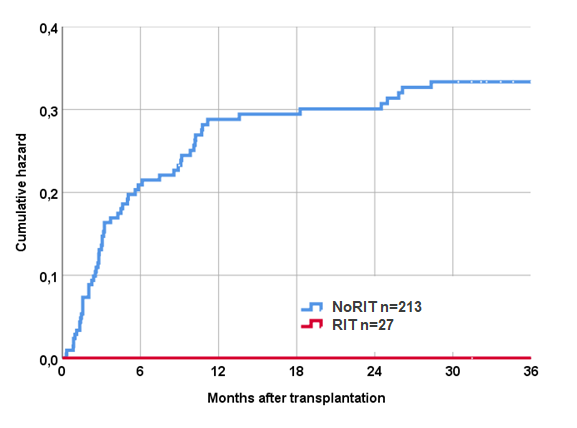
Conclusion: Based on the findings in this study, we conclude that single-dose rituximab induction in paediatric KT appears safe and to effectively prevent EBV viremia long-term. We therefore postulate that rituximab induction could be a forceful strategy to significantly reduce the risk of PTLD in paediatric solid organ recipients. Yet, in line with RCTs in adult KT, rituximab may have limited potential as inhibitor of early acute rejection. To fully appreciate the effects of rituximab induction in paediatric KT, prospective trials are needed.
Scandiatransplant. Swedish Kidney Foundation. Swedish Order of Freemasons.

right-click to download
