Esther Bernaldo-de-Quirós, Spain has been granted the TTS-SET International Transplantation Science Mentee-Mentor Awards
Preliminary results of the first clinical trial to prevent graft rejection in heart transplant children employing a cellular therapy with autologous Treg obtained from thymic tissue (thyTreg)
Esther Bernaldo-de-Quirós1, Manuela Camino2, Juan Miguel Gil-Jaurena3, Nuria Gil2, Rocío López-Esteban1, Marta Martínez-Bonet1, Diana Hernández-Flórez1, María Eugenia Fernández-Santos4, Laura Butrageño5, Megan K Levings6, Esme I Dijke7, Lori J West7, Marjorie Pion1, Rafael Correa-Rocha1.
1Laboratory of Immune-Regulation, Instituto de Investigación Sanitaria Gregorio Marañón (IISGM), Madrid, Spain; 2Pediatric Heart Transplant Unit, Hospital Gregorio Marañón, Madrid, Spain; 3Pediatric Cardiac Surgery Unit, Hospital Gregorio Marañón, Madrid, Spain; 4GMP Cell Production Unit, Instituto de Investigación Sanitaria Gregorio Marañón (IISGM), Madrid, Spain; 5Pediatric Intensive Care Unit, Hospital Gregorio Marañón, Madrid, Spain; 6Childhood Diseases Research Theme, BC Children's Hospital, Vancouver, BC, Canada; 7Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada
Introduction: Immune allograft rejection remains the main obstacle to successful transplants. Due to their suppressive capacity, regulatory T cell (Treg) therapy is a very promising alternative to prevent rejection and achieve indefinite graft survival. Several clinical trials conducted in adults with peripheral blood Treg demonstrate the safety of the therapy, although its efficacy seems to be limited. To overcome the current barriers and make this therapy effective, we explore a pioneering strategy that employs, as an alternative source of Treg cells, the thymic tissue discarded in pediatric cardiac surgeries.
Materials and methods: We developed a novel GMP-compatible protocol to obtain large numbers of Treg cells from pediatric thymic tissue (thyTreg) suitable for use in humans. After confirming the high quality of our therapeutic product and completing preclinical studies, we have started the first worldwide phase I/IIa clinical trial (NCT04924491) that evaluates the safety, feasibility and efficacy of autologous thyTreg in preventing rejection in children undergoing heart transplantation. We employ the thymic tissue removed in heart transplantation surgery to produce therapeutic doses of GMP thyTreg. At day +8±2 post-transplant, a single dose of fresh autologous thyTreg is administered to the patient intravenously (randomized to 10x106 or 20x106 cells/kg), and the remaining thyTreg are cryopreserved. Patients receive their usual immunosuppressants regime (tacrolimus, mycophenolate, short-term corticosteroids) and are clinical and immunological followed up to 2 years post-transplant. A cohort of heart transplant children receiving the same immunosuppressive regime without thyTreg administration is also recruited as the control group.
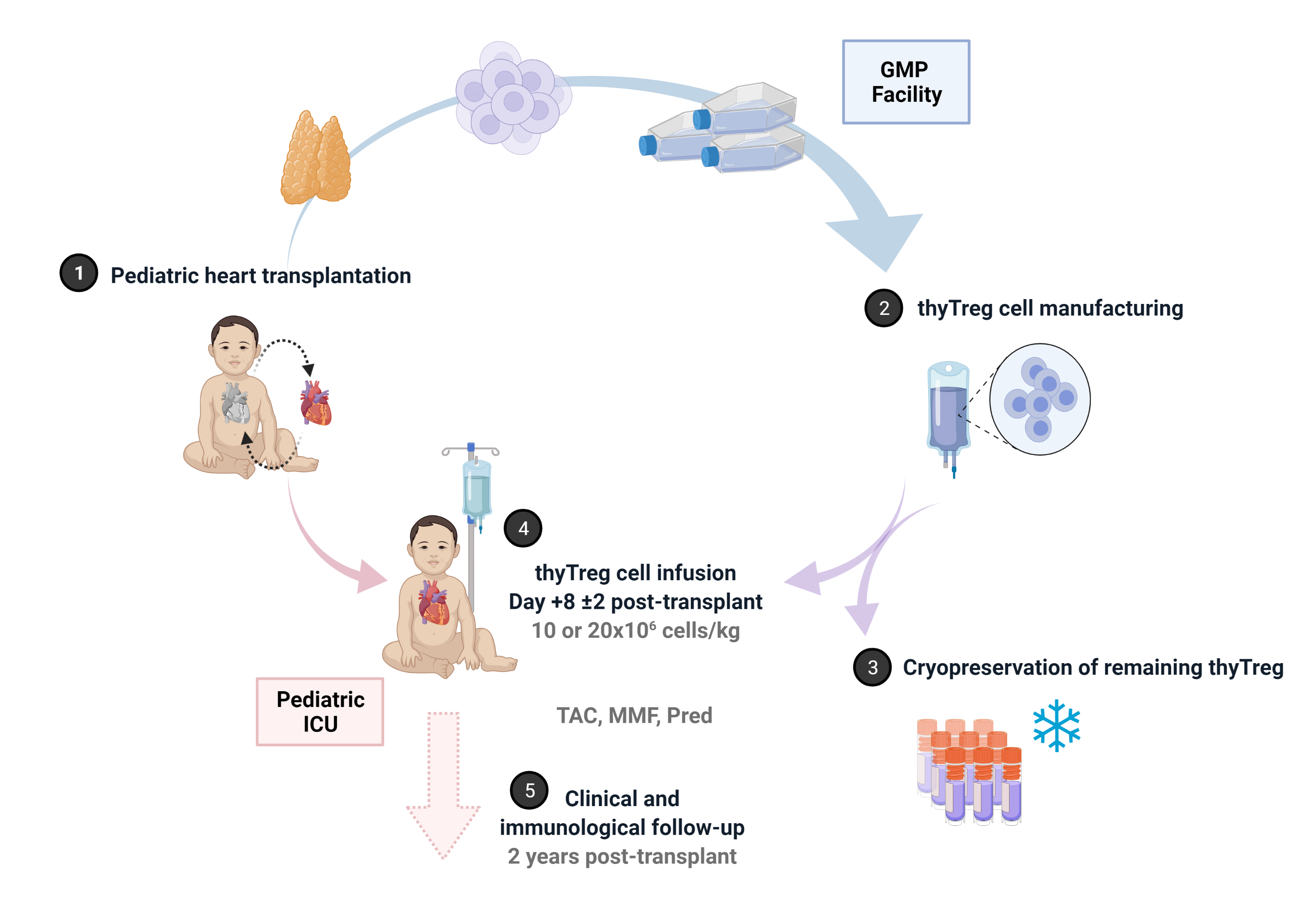
Results: To date, 4 patients have received a thyTreg dose. The four thyTreg products infused showed very high viability (>94%) and purity (CD25+FOXP3+) ≥85% in all cases. The procedure's safety is confirmed, and the preliminary results obtained in the children treated with this therapy are very encouraging. These results suggest that with a single infusion of thyTreg it is possible to maintain the reserve of Treg cells crucial to prevent rejection, even in these patients who have undergone thymectomy and treated with immunosuppressive therapy. Furthermore, so far, we have yet recruited six more patients in the trial who are still on the heart transplant waiting list, completing the 10 patients planned for this clinical trial.
Conclusion: ThyTreg cell constitutes a new therapeutic strategy to prevent rejection in children who have received heart transplants. Preliminary results of our clinical trial confirm the approach's safety and suggest that it can preserve the peripheral Treg pool. We are confident that the improved quality and amount of thyTreg obtained with this protocol could be a step to induce immunological tolerance and thus achieve the indefinite survival of the transplanted organ.
This work was supported by grants from Fundación Familia Alonso (FFAFIBHGM 2019), grants from Instituto de Salud Carlos III (ISCIII) co-financed by FEDER funds (PI21/00189; ICI20/00063; PI18/00495; PI18/00506) and from Comunidad de Madrid (EXOHEP-CM, B2017/BMD-3727).

right-click to download
