Clinical validation of a plasma donor-derived cell-free DNA assay to detect allograft rejection and injury in lung transplant
Justin P. Rosenheck1, David J. Ross2, Mena Botros1, Alexander Wong3, Jonathan Sternberg2, Yen-An Chen4, Nathan Liang4, Amy Baer4, Ebad Ahmed4, Ryan K. Swenerton4, Bernhard G. Zimmermann4, Gordon Fehringer2, Zachary P. Demko2, Michael Olymbios2, Cesar Escrig2, Paul R. Billings2, Brian C. Keller1.
1Division of Pulmonary, Critical Care and Sleep Medicine, The Ohio State University, Columbus, OH, United States; 2Medical Affairs, Natera, Inc., San Carlos, CA, United States; 3Department of Internal Medicine, The Ohio State University, Columbus, OH, United States; 4Research and Development, Natera, Inc., San Carlos, CA, United States
Background: Lung transplant patients are vulnerable to various forms of allograft injury, whether from acute rejection (AR) [encompassing acute cellular (ACR) and antibody-mediated rejection (AMR)], chronic lung allograft dysfunction (CLAD), or infection (INFXN). Previous research indicates that donor-derived cell-free DNA (dd-cfDNA) is a promising non-invasive biomarker for the detection of AR and allograft injury. Our aim was to validate a clinical plasma dd-cfDNA assay for detection of AR and other allograft injury and to confirm and expand on dd-cfDNA and allograft injury associations observed in previous studies.
Methods: We measured dd-cfDNA fraction using a novel SNP-based assay in prospectively collected plasma samples paired with clinical-pathologic diagnoses. dd-cfDNA fraction was compared across clinical-pathologic cohorts: stable, ACR, AMR, isolated lymphocytic bronchiolitis, CLAD/neutrophilic-responsive allograft dysfunction (CLAD/NRAD), and INFXN. Performance characteristics were calculated for AR and combined allograft injury (AR+CLAD/NRAD+INFXN) versus the stable cohort.
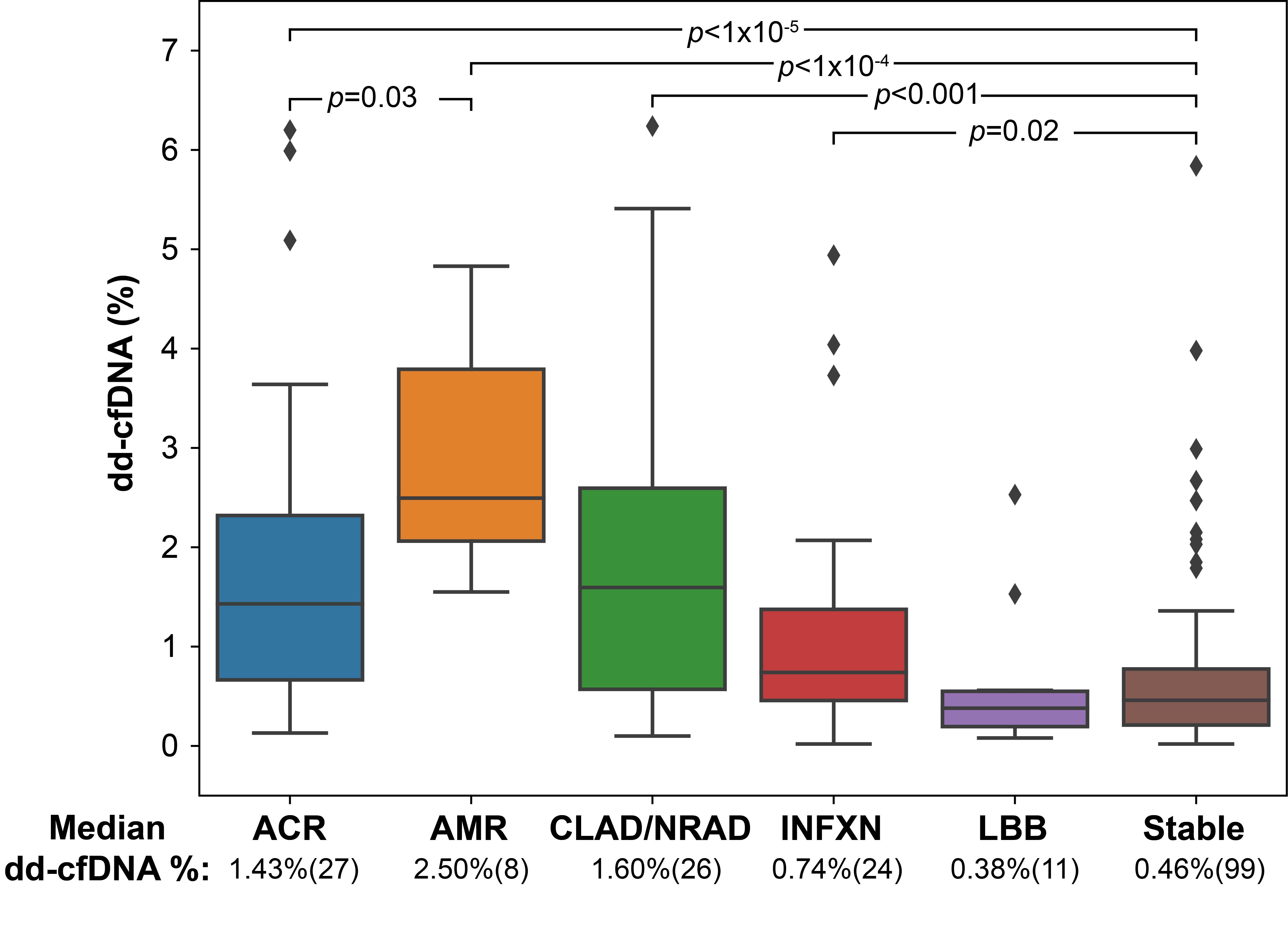
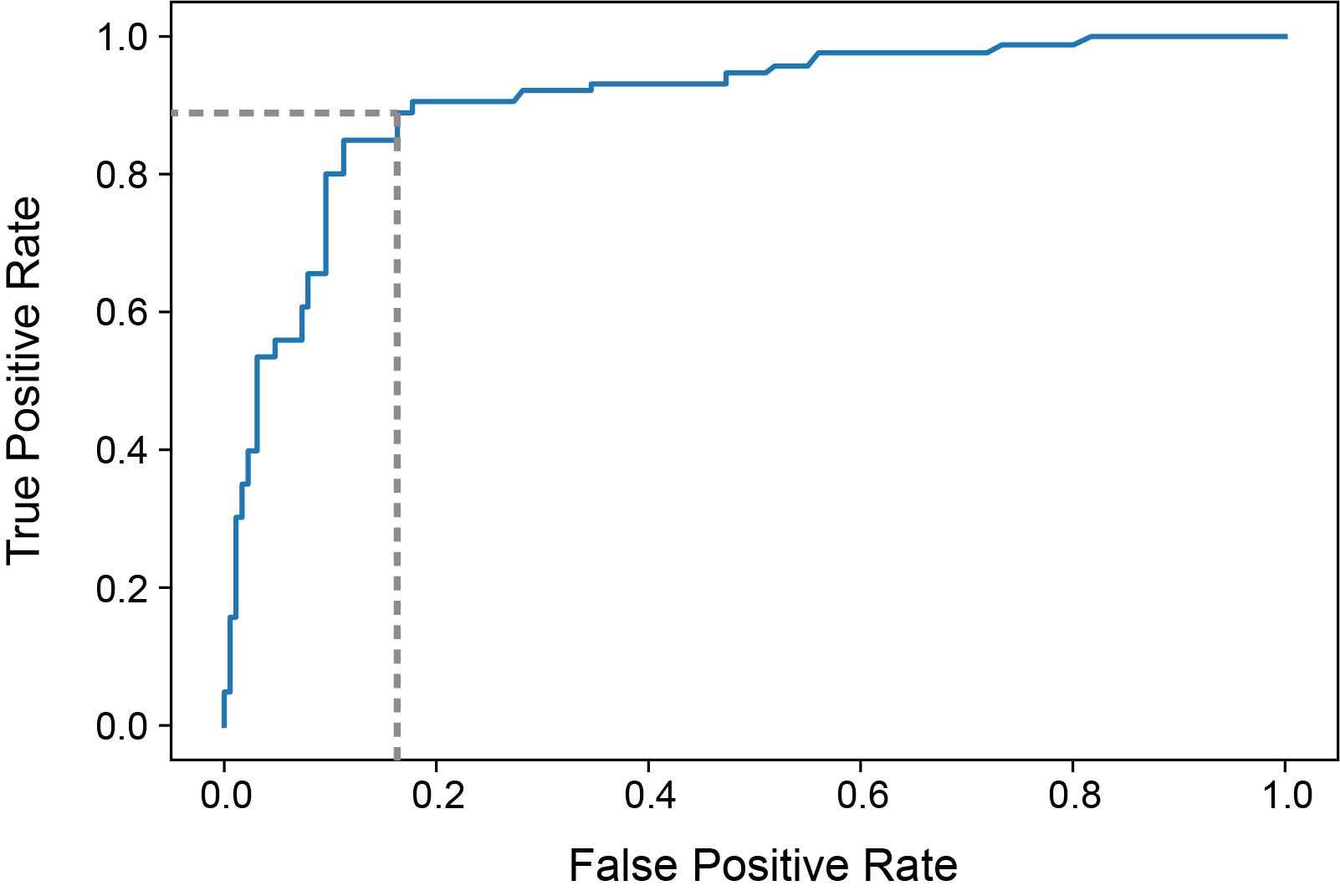
Results: The study included 195 samples from 103 patients. Median dd-cfDNA fraction was significantly higher for ACR (1.43%, IQR:0.67-2.32%, p=5x10-6), AMR (2.50%, IQR:2.06-3.79%, p=2x10-5), INFXN (0.74%, IQR:0.46-1.38%, p=0.02) and CLAD/NRAD (1.60%, IQR:0.57-2.60%, p=1.4x10-4) versus the stable cohort (0.46%, IQR:0.21-0.78%) (Figure1). Area under the receiver operator characteristic curve (AUROC) for AR versus stable was 0.91 (95% CI:0.83-0.98) (Figure 2). Using a ≥1% dd-cfDNA fraction threshold, sensitivity for AR was 89.1% (95% CI:76.2-100.0%), specificity 82.9% (95% CI:73.3-92.4%), positive predictive value (PPV) 51.9% (95% CI:37.5-66.3%), and negative predictive value (NPV) 97.3% (95% CI:94.3-100%). For combined allograft injury vs. stable AUROC was 0.76 (95% CI:0.66-0.85), sensitivity 59.9% (95% CI:46.0-73.9%), specificity 83.9% (95% CI:74.1-93.7%), PPV 43.6% (95% CI:27.6-59.6%) and NPV 91.0% (95% CI:87.9-94.0%).
Conclusion: These results indicate that dd-cfDNA can detect AR and other allograft injury. dd-cfDNA monitoring, accompanied by standard clinical assessments, represents a valuable precision tool to support lung transplant health.

right-click to download
