
Safety of once-weekly dulaglutide on post-transplant diabetes mellitus: a preliminary single-centre experience
Alessandro Mattina1, Maria Ausilia Giusti1, Salvatore Gruttadauria 2, Michele Pilato 3, Patrizio Vitulo 3, Margherita Occhipinti 4, Giovanni Zito5, Valentina Agnese 5, Pier Giulio Conaldi5, Diego Bellavia5.
1Department of Diagnostic and Therapeutic Services, IRCCS-ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione), Palermo, Italy; 2Department for the Treatment and Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione), Palermo, Italy; 3Department for the Treatment and Study of Cardiothoracic Diseases and Cardiothoracic Transplantation, IRCCS-ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione), Palermo, Italy; 4Diabetologic Unit, Medical Dept., Versilia Hospital, North West Tuscany, Lido di Camaiore (LU), Italy; 5Research Department, IRCCS-ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione), Palermo, Italy
Introduction: Almost all immunosuppressive drugs (IS), including glucocorticoids, calcineurin and mTOR inhibitors, play a central role in the development of Post-Transplant Diabetes Mellitus (PTDM). IS potentially cause the development of a dysglycemic state, both for the direct cytotoxic action on pancreatic beta cells and for the induction of insulin resistance. PTDM is also associated with increased risk for cardiovascular disease and with worse graft and patients’ survival. Currently there are no clear recommendations on the choice of the most safe and effective anti-diabetic drug for PTDM. IS are linked with several pharmacokinetic interactions and burdened with vary degree of nephrotoxicity. Glucagon like peptide- 1 receptor agonists (GLP1RA) are largely used for the treatment of type 2 diabetes. They act stimulating endogenous insulin secretion in a glucose-dependent manner. GLP1RAs reduce blood sugar by stimulating insulin release, suppressing high glucagon levels, delaying gastric emptying, and reducing food intake. Furthermore, GLP1 has been shown to promote proliferation of beta cells in animal models. GLP1RA has proven to be safe and effective in reducing HbA1c without directly causing hypoglycemia and has shown a clear association with cardiovascular risk reduction and renal protection. Despite GLP1RA seems to act with a mechanism that perfectly contrasts the pathogenetic aspects of PTDM, in the literature clinical experience is entirely limited to case series and retrospective studies.
Method: In order to assess primarily safety of GLP1RA therapy in patients with PTDM we have prospectively enrolled a sample of N = 20 patients referred to liver (N = 10), renal (N = 5), as well as heart (N = 4) and lung (N = 1) transplant at ISMETT. All patients were placed on once-weekly dulaglutide therapy. Weight, HbA1c Level, as well as complete lipid profile and liver function tests were performed at 3 months and again at 6 months. One-way Repeated measurements ANOVA was used to test significant change in collected variables over time.
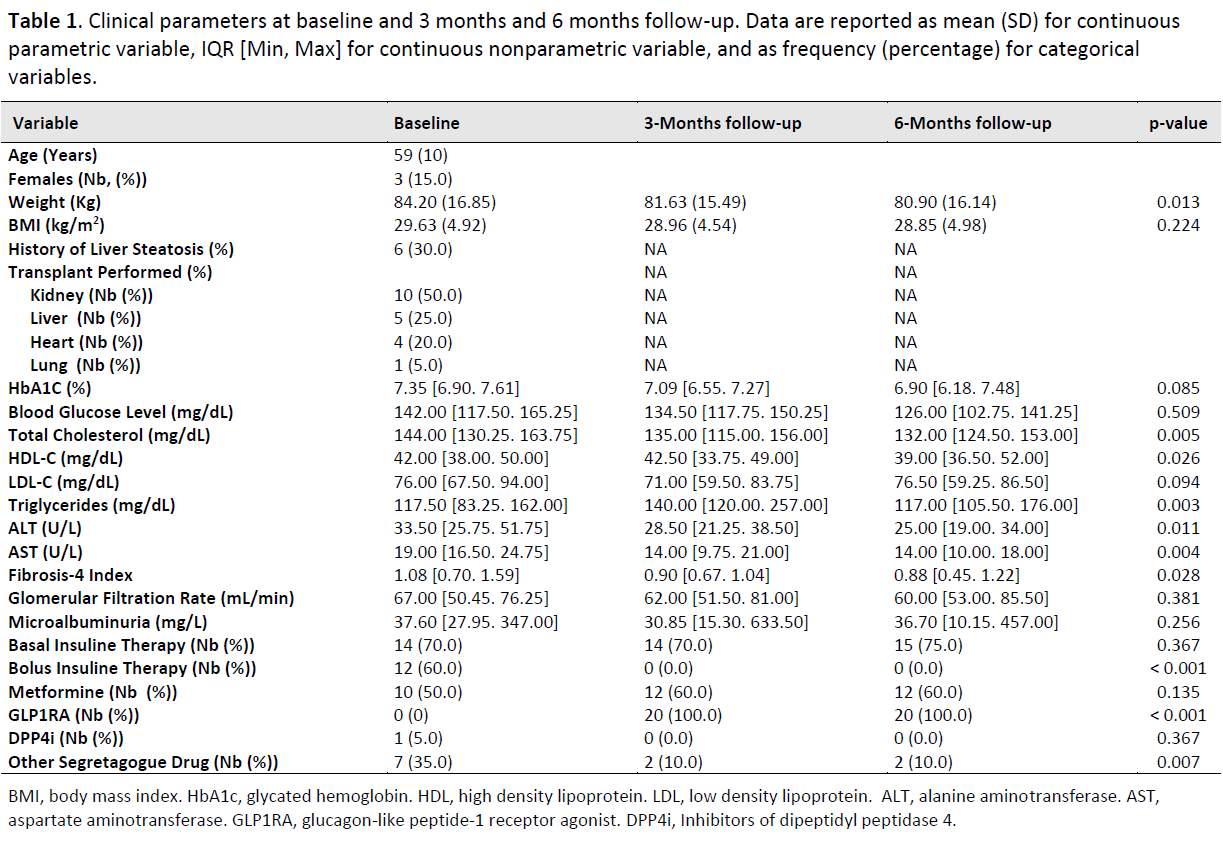
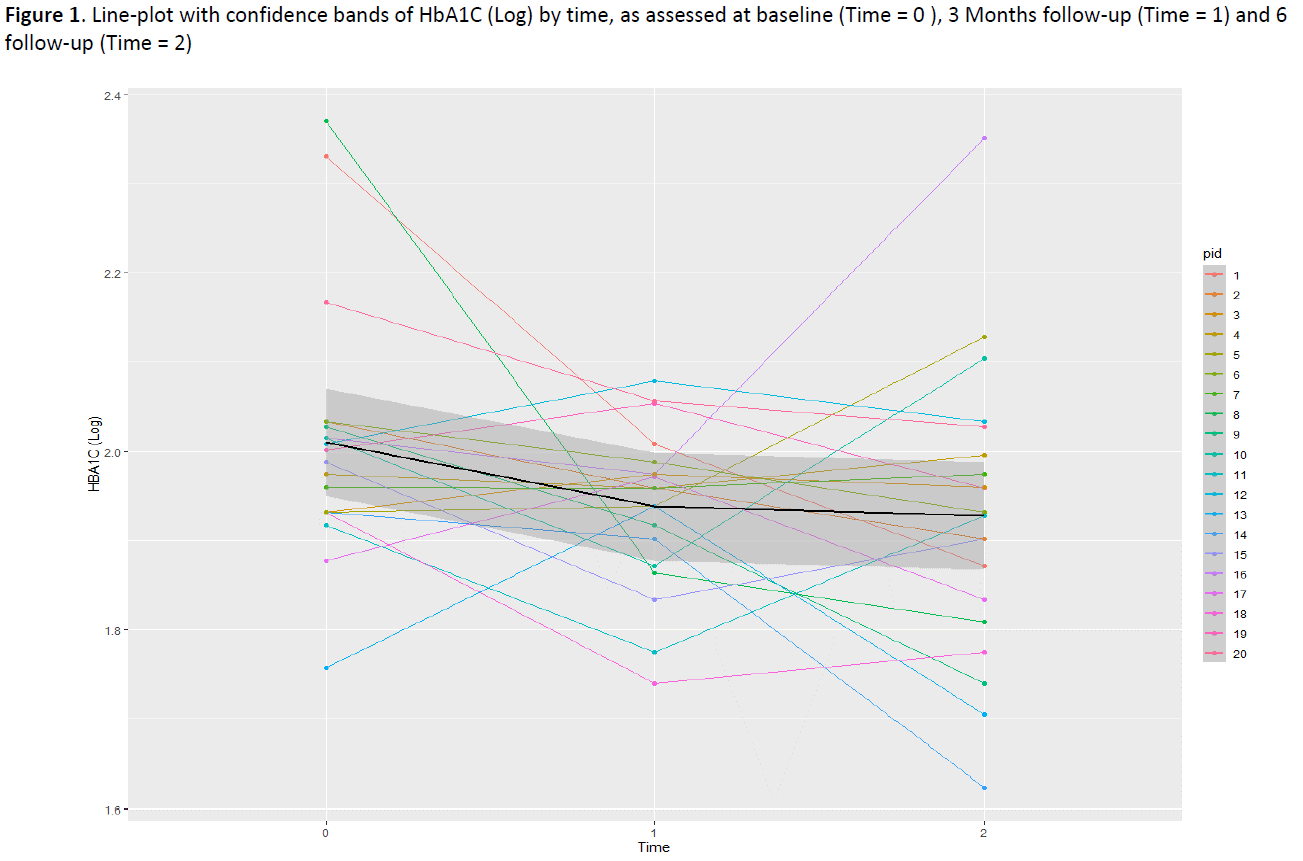
Results: The study was closed at 6 months follow-up visit. Mild gastrointestinal symptoms were experienced by patients at the treatment start and resolved in 2 weeks. There were no persistent side effects due to dulaglutide in our study population and all recruited patients were on dulaglutide at 6 months evaluation. Body weight, Total cholesterol, Fibrosis-4 Index, alanine aminotransferase and aspartate aminotransferase levels were all reduced at the end of the follow-up, while blood glucose as well as HbA1c were stable over time (Table 1, Figure 1).
Conclusion: Dulaglutide has proven to be safe in a population of transplanted patients with PTDM. In addition, a significative reduction of metabolic parameters was found. GLP1RA could be a preferred treatment for patients with PTDM considering also the extraglycemic effects of cardiovascular and renal protection.

right-click to download
