Aileen C Johnson, United States has been granted the Young Investigator Congress Scientific Award
Every 2-month belatacept maintenance therapy in kidney transplant recipients: 3-year follow-up of a randomized, non-inferiority trial
Aileen Johnson1, Geeta Karadkhele1, Neeta Shenvi2, Kirk Easley2, Christian P Larsen1, Idelberto R Badell1.
1Emory Transplant Center, Emory University, Atlanta, GA, United States; 2Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, GA, United States
Introduction: Maintenance immunosuppression with belatacept following kidney transplantation results in improved long-term graft function as compared to calcineurin inhibitors. However, broad application of belatacept has been limited, in part related to logistical barriers surrounding a monthly (q1m) IV infusion requirement. Our center conducted a randomized, non-inferiority trial comparing every 2-month (q2m) dosing to standard q1m maintenance. At twelve month follow up, q2m dosing was noninferior to standard q1m dosing as determined by renal function (eGFR). Two-month dosing was safe and well tolerated without a significant incidence of immunologic events in subjects adherent to the study protocol, but longer term follow-up is of interest to better elucidate patient and graft outcomes. Here, we present the 3-year outcomes from this study.
Methods: Low immunologic risk, stable renal transplant recipients a minimum of 1 year post-transplant were randomized to q1m or q2m belatacept maintenance therapy. 36-month follow-up was conducted as intention-to-treat on the population that initiated the study protocol. An autoregressive model using baseline-adjusted means with a random effect for subject was used to compare renal function between groups. Kaplan Meier survival analysis was performed for analysis of adverse and immunologic events.
Results: 163 patients initiated the study protocol and received treatment in the q1m control group (n=82) or q2m study group (n=81). Renal allograft function as measured by baseline adjusted eGFR was not significantly different between groups at 36 months (mean [95% CI], q1m: 73.43[70.37, 76.49] vs. q2m: 72.46[69.08, 75.84].
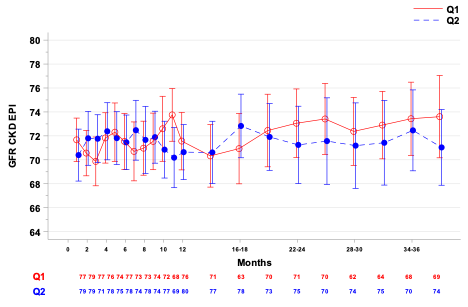
There were no statistically significant differences in time to death or graft loss (cumulative survival at 36 months, q1m: 92.6%, q2m: 95.0%, p=0.49; freedom from rejection (q1m: 98.7%, q2m: 92.4%, p=0.06), or freedom from DSA (q1m: 98.7%, q2m: 92.4%, p=0.06). During the extended 12-36 month follow-up period, 3 deaths, 1 graft loss occurred in the q1m group, compared to 2 deaths, 2 graft losses in the q2m group. In the q1m group, one patient developed DSA and acute rejection. In the q2m group, 3 patients developed DSA, 2 associated with acute rejection. All acute rejections in both groups were associated with documented medication nonadherence.
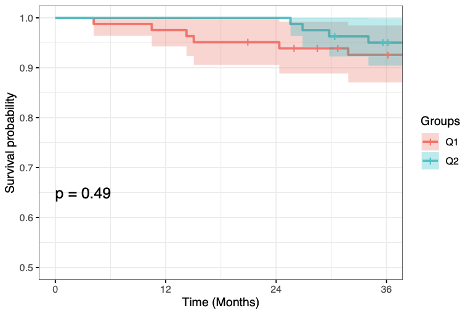
Conclusions: Based on similar renal function and adverse event rates at 36 months compared to q1m, q2m belatacept is a viable and safe maintenance immunosuppressive strategy in low immunologic risk kidney transplant recipients with potential to facilitate increased clinical utilization of costimulation blockade-based immunosuppression. (ClinicalTrials.gov NCT02560558)
James O. Robbins Foundation. James M. Cox Foundation. Carlos and Margeurite Mason Trust.

right-click to download
