Porcine ULBP1 disruption does not significantly reduce human NK cell activation and cytotoxicity in an in vitro model
Kevin Lopez1, Arthur A Cross-Najafi1, Kadir Isidan1, Yujin Park1, Jonathan A Fridell1, Wenjun Zhang1, Ping Li1, Burcin Ekser1.
1Division of Transplant Surgery, Department of Surgery, Indiana University School of Medicine, Indianapolis, IN, United States
Background: Pig-to-human xenotransplantation has the potential to address the critical organ shortage. Genetically-engineered pig organs have significantly prolonged survival in human and nonhuman primates by overcoming hyperacute rejection. Human natural killer (NK) cell-mediated acute xenograft rejection of porcine endothelial cells has been established. Porcine UL-Binding Protein (ULBP1) has been identified as a porcine ligand of human NK cell activating receptor NKG2D; interactions presumably lead to NK cell activation and ultimately porcine cell cytotoxicity. We sought to improve pig-to-human compatibility by eliminating ULBP1 ligand in an immortalized porcine liver-derived endothelial cell line (ipLDEC) with five-gene knockout (5GKO). The 5GKO cell line has mutations in GGTA1/CMAH/β4galNT2/SLA-I α-chain/β2M: three genes encoding enzymes responsible for xenoantigen production and two genes encoding swine leukocyte antigen class I.
Methods: CRISPR/Cas9 technology was used to knockout the porcine ULBP1 gene in the 5GKO cell line to create a ULBP1-KO/5GKO cell line. Human peripheral blood mononuclear cells (PBMCs) from four donors were activated with human IL-2 for five days. Human PBMCs were co-cultured with 5GKO, ULBP1-KO/5GKO, and Wild-Type (WT) ipLDECs. Cells were stained with antibodies to CD45, CD3, CD56, and CD107a. Flow cytometry analysis was gated on the CD3-CD56+ NK cell population and monitored for expression of CD107a, a marker of NK cell degranulation. Calcein AM cytotoxicity assay was performed to determine human NK cell-mediated ipLDECs cell death. WT, 5GKO, ULBP1-KO/5GKO, HLA-G+/5GKO (positive control) were incubated in a calcein solution for 30 minutes at a concentration of 106 cells/mL. Stained porcine cells were co-cultured with activated human PBMCs at a 10:1 E:T ratio for 4 hours. Co-culture supernatant was harvested and calcein release was measured at an excitation wavelength of 485 nm and emission wavelength of 530 nm to calculate cytotoxicity percentages.
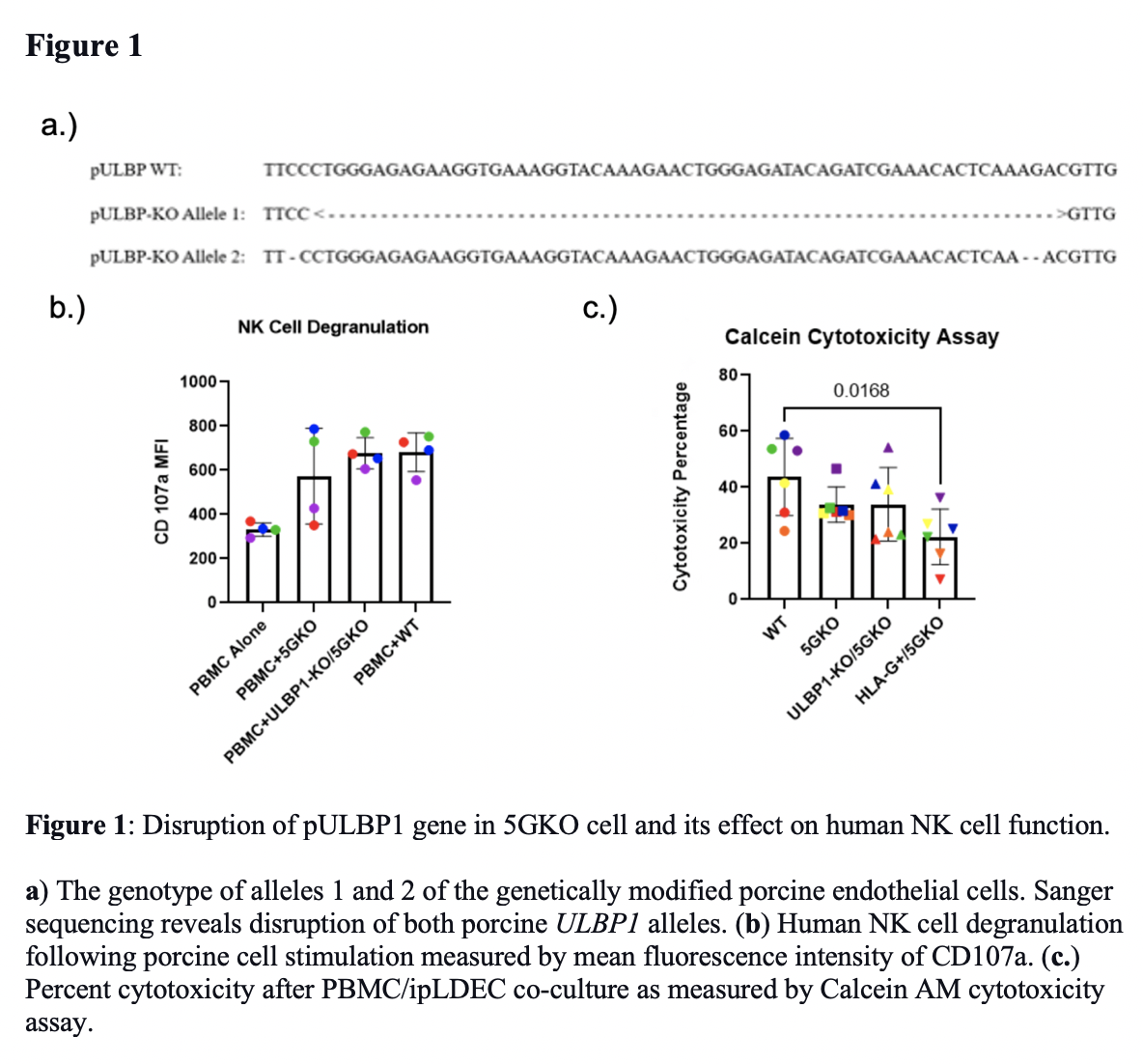
Results: Porcine ULBP1 gene mutation was confirmed by Sanger sequencing (Figure 1a), establishing a porcine ULBP1-KO/5GKO cell line. There is no statistically significant difference in NK cell activation between co-cultures of human PBMCs (n=4) with pULBP1-KO/5GKO and 5GKO ipLDEC using CD107a. (Figure 1b). There is no statistically significant difference in NK-induced cytotoxicity between co-cultures of human PBMCs (n=6) with porcine ULBP1-KO/5GKO and 5GKO cell lines using Calcein AM assays (Figure 1c). As expected, HLA-G+/5GKO cytotoxicity was decreased (p = 0.0168) compared to WT (Figure 1c).
Conclusion: We established a porcine ULBP1-KO/5GKO cell line. Our studies demonstrate no statistically significant difference in NK cell activation and NK cell-mediated cytotoxicity between 5GKO and ULBP1-KO/5GKO cell lines. Our studies show that ULBP1 is not a crucial ligand for human NKG2D. Future studies will target different human NK cell activating porcine ligands.

right-click to download
