
Carla C. Baan, PhD, is Professor and head of the Nephrology & Transplantation Laboratory at Erasmus Medical Center, University Hospital Rotterdam, The Netherlands. Dr Baan obtained her doctorate from Erasmus University, The Netherlands.
Her position involves the supervision of doctorate research related to the role of cytokines, regulatory T cells, B cells, cell therapy and immunosuppressive drugs in clinical organ transplantation. The aim of her translational work is to titrate the immunosuppressive burden on our patients in such a way that side-effects (infections, malignancies, cardiovascular events) are kept at a minimum while at the same time rejection processes are prevented.
Dr Baan has been the President of the European Society for Organ Transplantation (2011-2013) and is a member of the board of the International Society for Heart and Lung Transplantation. Currently, dr Baan is the Executive Editor_Basic Sciences and the Social Media Editor of the Transplantation Journal.
Novel avenue of allograft monitoring: direct measurement of donor-derived extracellular vesicles in human plasma
Wouter Woud1, Dennis A Hesselink1, Martin J. Hoogduijn1, Carla C Baan1, Karin Boer1.
1Erasmus MC Transplant Institute, Department of Internal Medicine, University Medical Center Rotterdam, Rotterdam, Netherlands
Background: Extracellular Vesicles (EVs) - regarded as “snapshots” of their cell of origin - represent promising liquid biomarkers to monitor allograft function post transplantation. Recently, we developed an imaging flow cytometry (IFCM) based protocol to identify and characterize EV ≤400 nm in molecularly complex samples such as human plasma without prior isolation of EVs. Using this protocol, we measure allograft derived EVs based on HLA phenotype as a first step to detect allograft specific EVs in the circulation of kidney transplant (KTx) recipients.
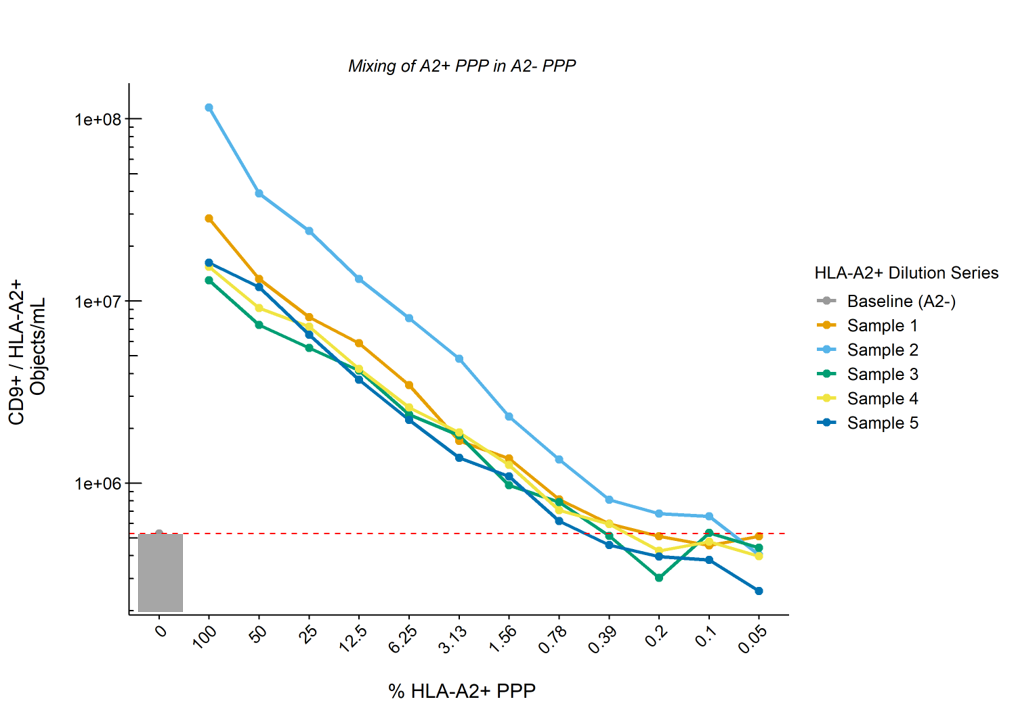
Materials & Methods: EDTA blood samples from kidney transplant donors (HLA-A2+, n=21) and recipients (HLA-A2-, n=33) were collected before transplantation as well as 3 days, 7 days, 6 months and during ‘for-cause’ biopsies (recipients only) after transplantation. Platelet-poor plasma (PPP) was stained with a donor-specific HLA antibody (HLA-A2) in combination with a common EV marker (tetraspanin CD9) and measured using standardized IFCM.
Results: Quantification and comparison of CD9+/HLA-A2+ double-positive EV showed 1.1E7 ± 8.9E6 vs 3.5E5 ± 2.5E5 objects/mL for donor and recipient (pre-KTx) EVs respectively, with recipients A2- EVs concentrations representing background level of the machine. CV values for inter- and intra-assay variability were 16% and 11%, respectively. Serial dilution of A2+ PPP in A2- PPP (n=5) showed a linear reduction in the numbers of CD9+/HLA-A2+ EVs according to the dilution rate whilst total CD9+ EV levels remained unchanged. The lower limit of detection of our protocol was defined as the dilution at which point CD9+/HLA-A2+ EVs dropped below baseline (A2- PPP), and was determined to be ~1% of the concentrations measured in undiluted A2+ PPP (Figure). Measurement of longitudinally collected recipient samples revealed the detection of allograft derived EVs as soon as 3 days – but up to at least 6 months – after KTx.
Conclusion: Here we demonstrate for the first time the detection of allograft derived EVs in the circulation of KTx recipients in unprocessed human plasma samples. Identification, quantification and characterization of these EVs opens up the possibility to monitor these EVs over time after transplantation, and may prove to be a minimally-invasive biomarker.

right-click to download
