Early post-operative thrombotic microangiopathy in deceased donor kidney transplant: case series
Adela Mattiazzi1, Ramon Seneriz Olivencia1, Mariana Velazquez3, Thomas Harrington2, Giselle Guerra1.
1Medicine/ Nephrology, University of Miami, Miami, FL, United States; 2Medicine/Hematology, University of Miami, Miami, FL, United States; 3Medicine, University of Miami, Miami, FL, United States
Background: Transplant associated thrombotic microangiopathy (TA-TMA) is well recognized as a serious complication of renal transplantation. TA-TMA is characterized by injury of arterioles and capillaries and encompasses syndromes of thrombocytopenia, microangiopathic hemolytic anemia(MAHA) and variable degrees of kidney graft dysfunction. TA-TMA can be classified into recurrent TMA (from underlying complement mutations) and de novo TMA. The incidence of de novo TMA ranges from 3-14%. Causes of de novo TMA include immunosuppressive drugs (tacrolimus, mTOR inhibitors, valgancyclovir), ischemia reperfusion injury, viral infections (CMV, Parvovirus B19, HIV, HHV-6) and antibody-mediated rejection. We have found that many of these cases also have very low levels of ADAMTS13 but do not meeting clinical criteria for TTP. If the latter is secondary to endothelial damage vs consumption process is still unknown.
Objective: The purpose of this study was to identify if any specific risk factors or variables are associated with TA-TMA in the setting of deceased donor kidney transplantation (DDKT).
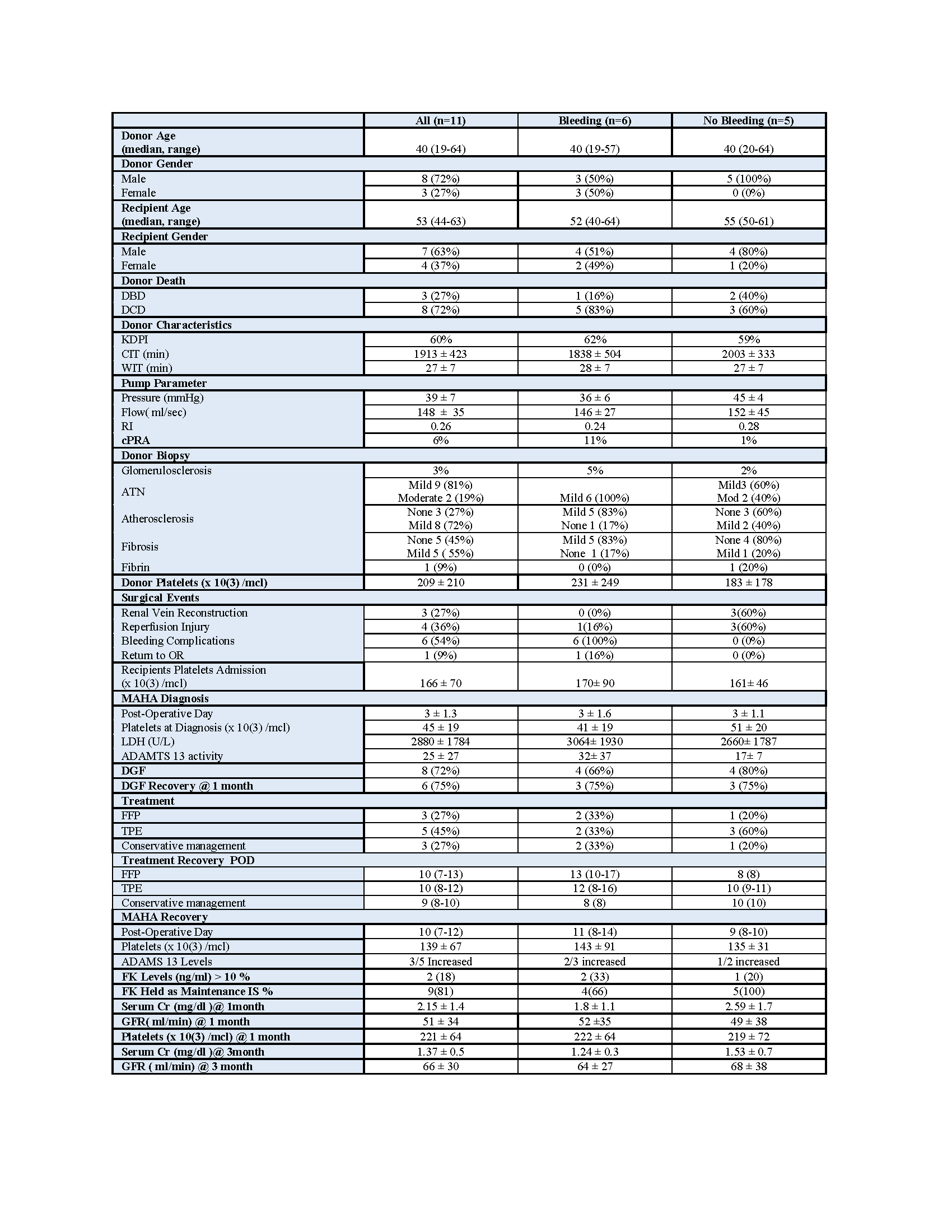
Methods: We retrospectively collected data on 11 patients who were diagnosed with TA-TMA in the immediate post operative period (defined as less than 2 weeks from transplant date) since January, 2017. We analyzed donor characteristics including age, type of deceased donor, last known platelet count, biopsy features, pump parameters, cold ischemia time (CIT) , warm ischemia time (WIT), reperfusion injury (RI) described at transplant surgery. Recipient variables included baseline platelet count on admission, the post-op day of diagnosis and at resolution (defined as platelet count > 100K and no need for additional transfusion), the use of thymoglobulin, ADAMS13 activity, changes in maintenance immunosuppression, evidence of post op bleeding, delayed graft function (DGF) and its recovery, and renal function at 1 and 3 months post transplant (Table).
Results: 72% of the kidneys were from donation after cardiac death donors (DCD) . The mean arterial pressure on the perfusion pump was 39 ± 7 mmHg and the flow was 148 ± 35 ml/sec. 81 % of the donor biopsies showed mild ATN, 72% had evidence of atherosclerosis and 54% of fibrosis and only one showed fibrin thrombi in the glomerulus. The mean CIT and WIT were 1913 ± 423 and 27 ± 32 min, respectively. The mean donor platelet count and baseline recipient's platelet count were 209 ± 210 and 166 ± 71 x 10(3) /mcl, respectively. The TA-TMA event occurred on post op day 3± 1.3 with a mean platelet count of 45 ± 19 x 10(3) /mcl. ADAMTS 13 level was <10% in 54% of the cases and LDH level 2880 ± 55 U/L. 3 patients received FFP infusions, 5 received plasmapheresis and 3 received conservative treatment. The microangiopathic hemolytic anemia recovered in 10 ± 2.4 days to a platelet count of 130 ± 67 x 10(3) /mcl. There were 6 cases of post op bleeding (55%) with 4 requiring surgical intervention and 4 cases of RI (36%). Of the 8 patients that sustained DGF, 6 recovered at one month, one recovered at 3 months post transplant (having history of low C3 and late biopsy proven TMA) and one died. The mean SCr and eGFR at 1 and 3 months were 2± 1.1, 51 ± 34 and 1 ± 0.5 mg/dl and 66± 30 ml/min, respectively.There were no statistical differences on the above parameters when the patients were grouped by the presence of post operatory bleeding . However, when we added the reperfusion injury factor, we found a lower ADAMTS 13 levels of 17 ±7 UI/dl in the non bleeding group who sustained RI (60%) vs 21± 37 UI/dl in the bleeding group who had only one patient with RI (17%) (p=0.24).
Conclusions: TA-TMA in the immediate postoperative period can be difficult to diagnose and treat. Our findings demonstrated that 55% of the cases were associated with post operatory bleeding leading to the reduction of ADAMTS 13 levels, most likely by platelet consumption. 36% were related to reperfusion injury where the consumption of ADAMTS 13 could be explained by direct endothelial damage. None of the patients needed other than conservative therapy. This data needs to be correlated in a larger cohort. In this context we are planning to prospectively study ADAMTS 13 activity in the immediate post kidney transplant period.

right-click to download
