
https://www.sydney.edu.au/medicine-health/about/our-people/academic-staff/jennifer-li1.html
Adoptive tolerogenic dendritic cell therapy protects against renal ischemia reperfusion injury
Jennifer Li1, Harry Robertson1, Andrew Mallett2,3,4, Stephen Alexander5, Philip O'Connell1, Natasha Rogers1,6.
1Centre for Transplant and Renal Research, Westmead Institute for Medical Research, Westmead, Australia; 2Institute for Molecular Biosciences , The University of Queensland, Brisbane, Australia; 3Department of Renal Medicine, Townsville University Hospital, Townsville, Australia; 4College of Medicine and Dentistry, James Cook University, Townsville, Australia; 5Department of Renal Medicine, Children's Hospital Westmead, Westmead, Australia; 6Department of Renal Medicine, Westmead Hospital, Westmead, Australia
Aim: Elucidate the pathways through which tolerogenic dendritic cell (TolDC) therapy provide protection against renal ischemia reperfusion injury (IRI).
Method: Ex-vivo TolDC (+/- lipopolysaccharide (LPS) stimulation) were derived from C57BL/6 bone marrow and cells were assessed for flow, functional (mixed lymphocyte reaction and TolDC-renal tubular epithelial cell (RTEC) co-cultures), and transcriptomic phenotype. Male, C57BL/6 mice underwent bilateral renal IRI (20 minutes/36°c) and treated perioperatively with adoptive cell therapy (live/CD11c-enriched TolDC or LPS-TolDC cells) or PBS control. Mice were also subjected to bilateral IRI (22minutes/36.5°c) to assess the impact of liposomal clodronate on cell therapy efficacy. Analysis of renal function, histology, biomolecular phenotyping, and spatial transcriptomics was performed 24-hours post-operatively.
Results: Gene set enrichment analysis (GSEA) revealed upregulated immune pathways in LPS-TolDC vs TolDC, supporting a semi-activated state. Elevated PDL1:CD86 MFI ratio (p<0.05), increased supernatant IL-10 with reduced IL-12p70 (p<0.001) and lymphocyte hypoproliferation was seen with both TolDC and LPS-TolDC compared to controls. Co-cultured RTECs had lower TNF-alpha/KIM-1/LCN2 mRNA expression (p<0.01) in response to LPS. Compared to controls, mice treated with LPS-TolDC, but not TolDC, were protected against AKI, with lower serum creatinine (p=0.006 vs p=0.28 respectively), histological injury and cell death scores (p<0.05) (Figure 1). Depletion of recipient myeloid cells with liposomal clodronate did not impair the reno-protective capacity of cell therapy (creatinine 30.6±19mmol vs 155±44.7mmol/L, p<0.001). LPS-TolDCs were more likely to localise to the kidney compared to TolDCs (7.7 vs 3.5 % CD45+, p<0.00) by flow cytometry tracking. Kidney mRNA revealed reduced pro-inflammatory and antioxidant expression (IL-6/TNF-alpha/CCL2/SOD/inducible-NOS, p<0.05), which was supported and further defined by distinct clusters shown via spatial transcriptomics. GSEA revealed reduced innate, adaptive and cell death pathways, with upregulation of antioxidant pathways in kidneys from mice who received LPS-TolDC therapy (Figure 2).
Conclusion: LPS-TolDCs demonstrate potent protection against renal IRI and is associated with reduced kidney inflammation, oxidative stress, and cell death. This evidence supports further investigation into cell therapy to modulate IRI severity.
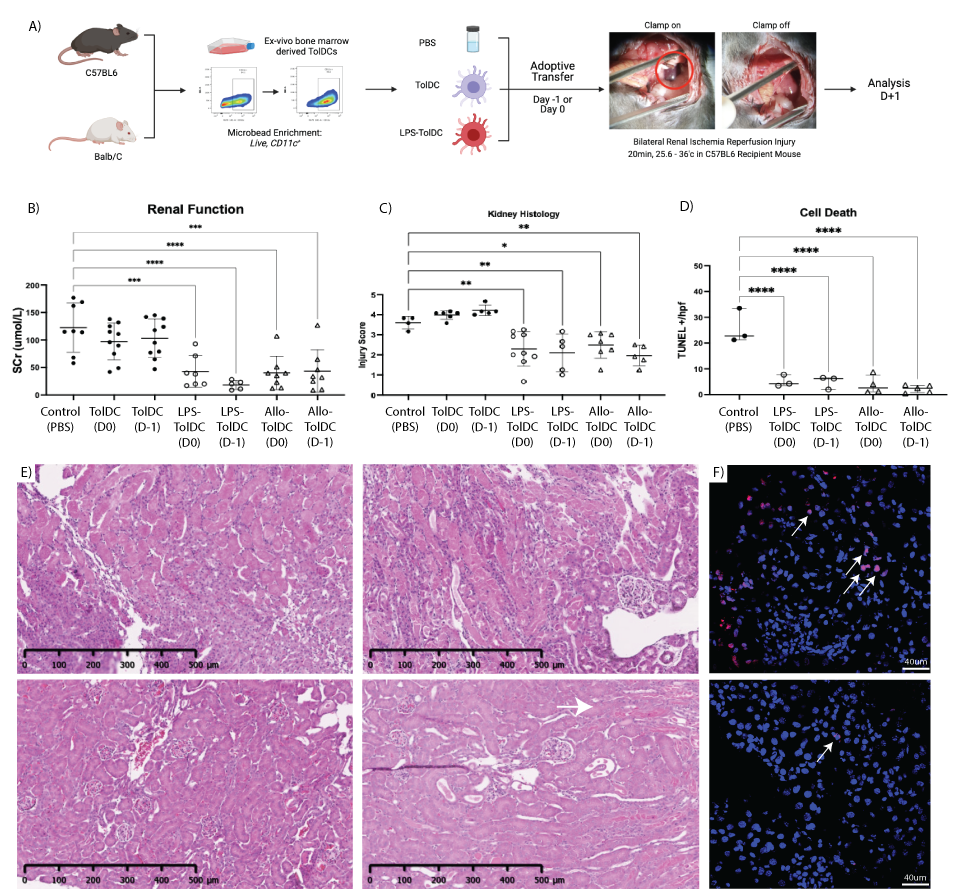
Figure 1: A) overview of adoptive cell therapy in renal IRI. B) Serum creatinine, C) H&E injury scores and D) TUNEL staining results between groups shown. Representative H&E images in E) from PBS and TolDC(top left and right); LPS-TolDC and AlloTolDC (bottom left and right) and F) TUNEL stains control (top) and LPS-TolDC(bottom) kidneys.
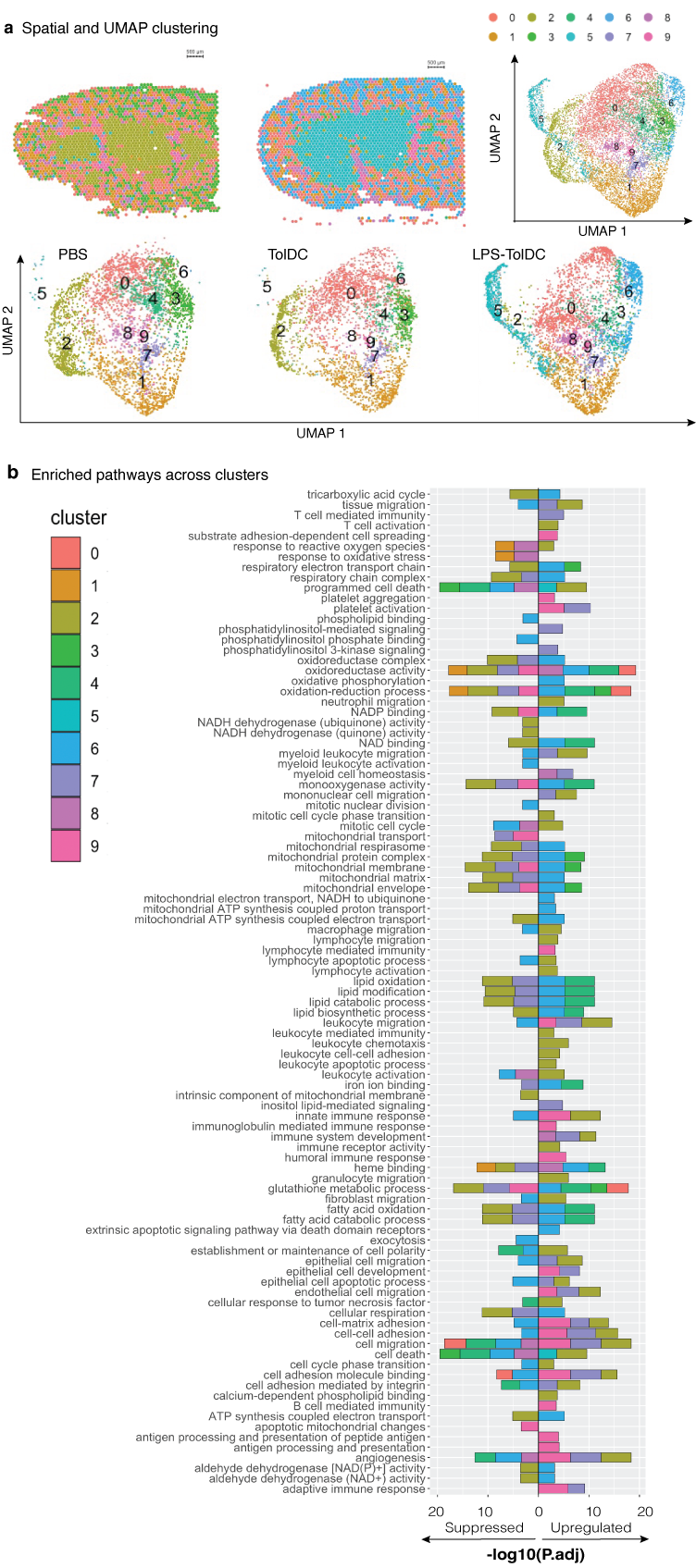
Figure 2: A) Spatial transcriptomic and UMAP projections from 6 mice kidneys (n=2 from PBS/TolDC/LPS-TolDC) and B) enriched pathways for each cluster in a cumulative bar graph.

right-click to download
