Necroptosis plays a role in acute kidney injury (AKI) and the progression to renal fibrosis following ischemia reperfusion injury (IRI)
Aspasia Pefanis1,2,3, Jennifer McRae1, Anjan Bongoni1, Frank Ierino2,3, Peter Cowan1.
1Immunology Research centre, St Vincent's Hospital, Melbourne , Australia; 2Department of Nephrology , St Vincent's Hospital, Melbourne , Australia; 3Department of Medicine, The University of Melbourne , Melbourne , Australia
Introduction: In kidney transplantation, ischemia reperfusion injury (IRI), a type of acute kidney injury (AKI), is associated with poorer outcomes including delayed graft function, rejection and chronic kidney disease (CKD) with fibrosis. Therapies to reduce AKI and CKD following transplantation have been investigated but none has been translated into clinical practice. Here we examined necroptosis, a pathway of regulated necrosis that has been implicated in kidney IRI. Necroptosis is triggered by recruitment of the intracellular kinases RIPK1 and RIPK3 and activation of the pseudokinase MLKL. Active MLKL causes cell death by plasma membrane rupture, driving “necroinflammation”. RIPK1 and RIPK3 may also have a cell death-independent role in the development of kidney fibrosis. The aims of this study were to (1) investigate the role of necroptosis in a mouse IRI model of AKI, using knockout mice and small molecule inhibitors, and (2) determine whether inhibition of RIPK1 or RIPK3 after AKI interferes with the progression to CKD.
Methods: AKI model: 10-12 week old male MLKL KO mice and wild type (WT) littermates underwent right nephrectomy followed by 18, 20, 22 or 24 min left kidney ischemia. Samples were collected at 24 h to assess renal injury, inflammation and necroptosis. In a separate experiment using the 18 min model, WT mice were treated either before or after IR with a RIPK1 inhibitor Nec-1s, a RIPK3 inhibitor GSK872, or vehicle.
CKD-after-AKI model: This model was adapted from the AKI 18 min model by (i) omitting right nephrectomy, (ii) delaying the endpoint to 28 days, and (iii) extending the analyses to include renal fibrosis (Masson’s trichrome staining) and relevant gene expression. WT mice were treated with Nec-1s, GSK872 or vehicle daily on days 3-9.
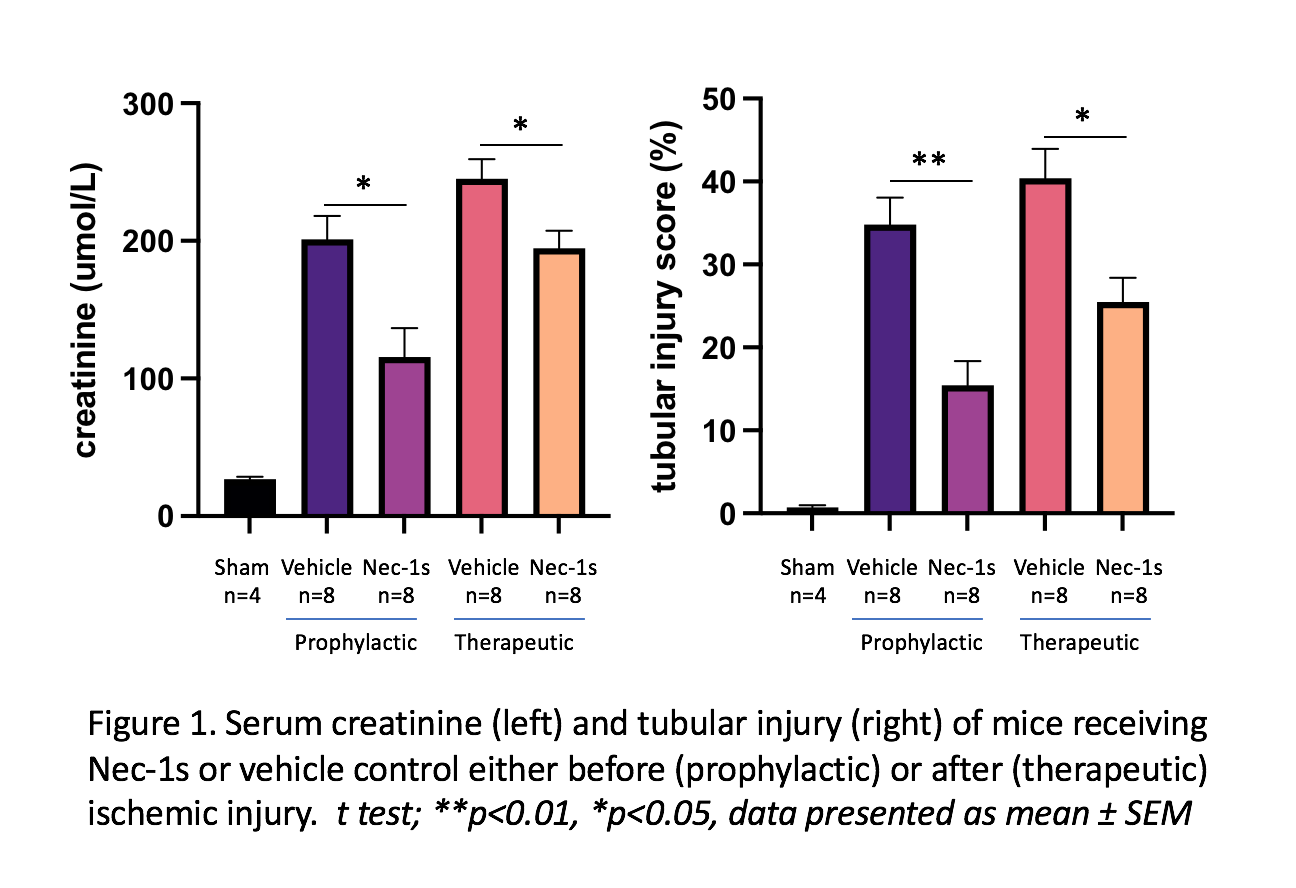
Results: In the AKI model, expression of necroptotic (RIPK1, RIPK3 and MLKL), kidney injury (KIM-1, NGAL) and inflammatory (IL-6, IL-1β, IL-33, TNFα) genes was significantly upregulated following moderate (18 min) and severe (20-24 min) ischemia. MLKL KO mice were protected from moderate but not severe IRI, with lower creatinine (µmol/L) (47.13±6.28 vs 80.78±10.45, p=0.007) and less tubular injury (%) (16.79±2.80 vs 26.95±3.68, p=0.048) compared to WT littermates. WT mice receiving Nec-1s before or after IR showed reduced creatinine and tubular injury compared to vehicle controls, whereas GSK872 was not protective.
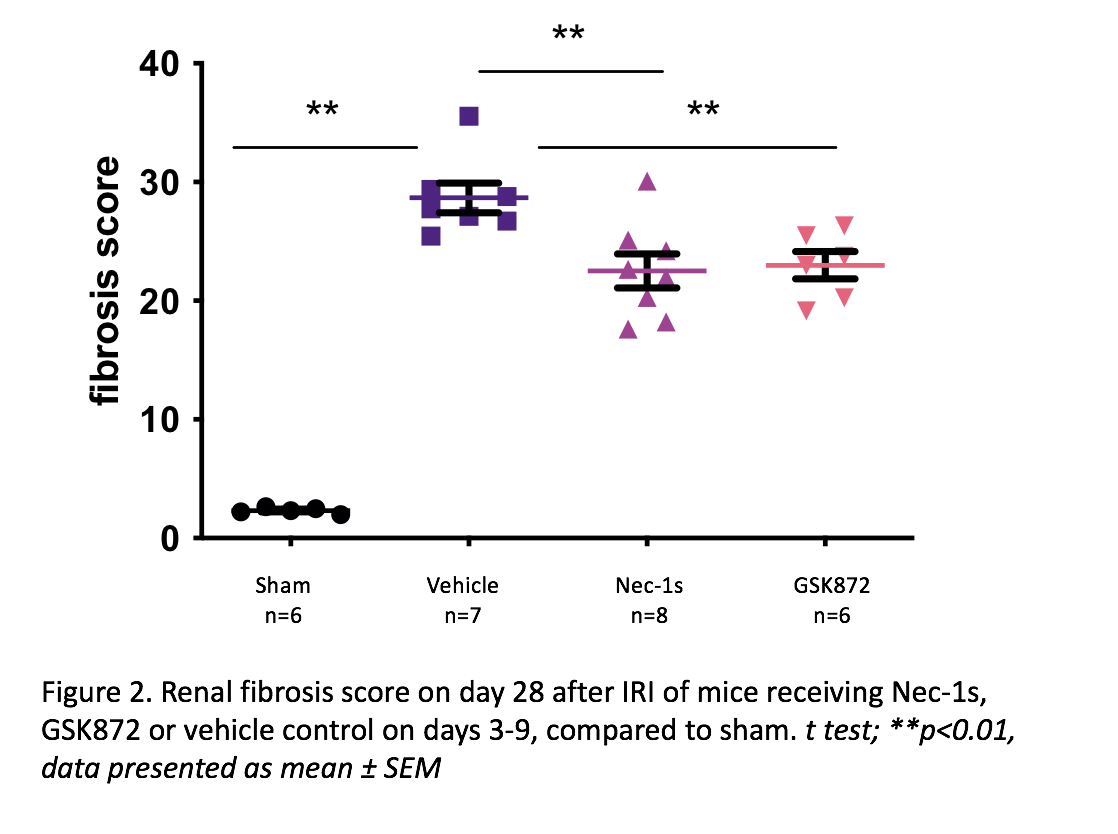
In the CKD-after-AKI model, vehicle-treated WT mice showed significant renal fibrosis at 28 days compared to sham (fibrosis score 28.68±1.25 vs 2.33±0.11, p=0.0025), with increased expression of RIPK1 (p=0.0079), RIPK3 (p=0.0025), and the pro-fibrotic genes TGFβ (p=0.0025), HIF1α (p=0.0025) and Col-I (p=0.0025). Inhibiting RIPK1 or RIPK3 on days 3-9 resulted in reduced fibrosis.
Conclusion: Our data support a role for necroptosis in AKI and fibrosis following IR, and identify inhibition of the necroptosis pathway as a potential therapeutic strategy.
NHMRC scholarship GNT1168307.

right-click to download
