Urinary extracellular vesicle DNA cargo reflects kidney allograft injury
Ivana Sedej1,3, Maja Štalekar2, Magda Tušek Žnidarič2, Katja Goričar3, Nika Kojc4, Polona Kogovšek2, Vita Dolžan3, Metka Lenassi3, Miha Arnol1.
1Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia; 2Department of Biotechnology and Systems Biology, National Institute of Biology, Ljubljana, Slovenia; 3Institute of Biochemistry and Molecular Genetics, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia; 4Institute of Pathology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Introduction: Following allograft injury, donor-derived cell-free DNA (dd-cfDNA) levels increase. Numerous research studies have demonstrated increased dd-cfDNA levels in blood and urine as biomarkers of kidney allograft injury in patients with transplanted kidney. Extracellular vesicles (EVs) are nanosized particles released by almost all cells. Because of the anatomical proximity of kidney EV-producing cells and urine, (dd)DNA containing urinary EVs (uEVs) are potentially relevant biomarkers of allograft injury. The aim of our study was to explore uEVs and their DNA cargo, to reveal their association with allograft injury and finally to compare their biomarker potential with (dd)cfDNA found directly in the urine of patients with transplanted kidney.
Method: We collected a second morning urine sample from 40 patients before surveillance or for-cause biopsy. We also collected blood and biopsy samples for donor-recipient genotyping. Considering histological analysis, we divided patients into normal histology, rejection injury (combination of antibody- (ABMR) and T-cell mediated rejection (TCMR)), and non-rejection injury group. First, we isolated uEVs (using protocol based on size-exclusion chromatography), their DNA cargo (uEV-DNA), and cfDNA from urine. We used nanoparticle tracking analysis to determine the concentration and size of uEVs. For DNA parameter analysis, including yield, copy number, integrity index, and donor-derived fraction, we used fluorometry and digital droplet PCR. We applied DNase assay and electron microscopy (TEM) immunogold labelling technique to investigate whether DNA was present inside or outside the uEVs.
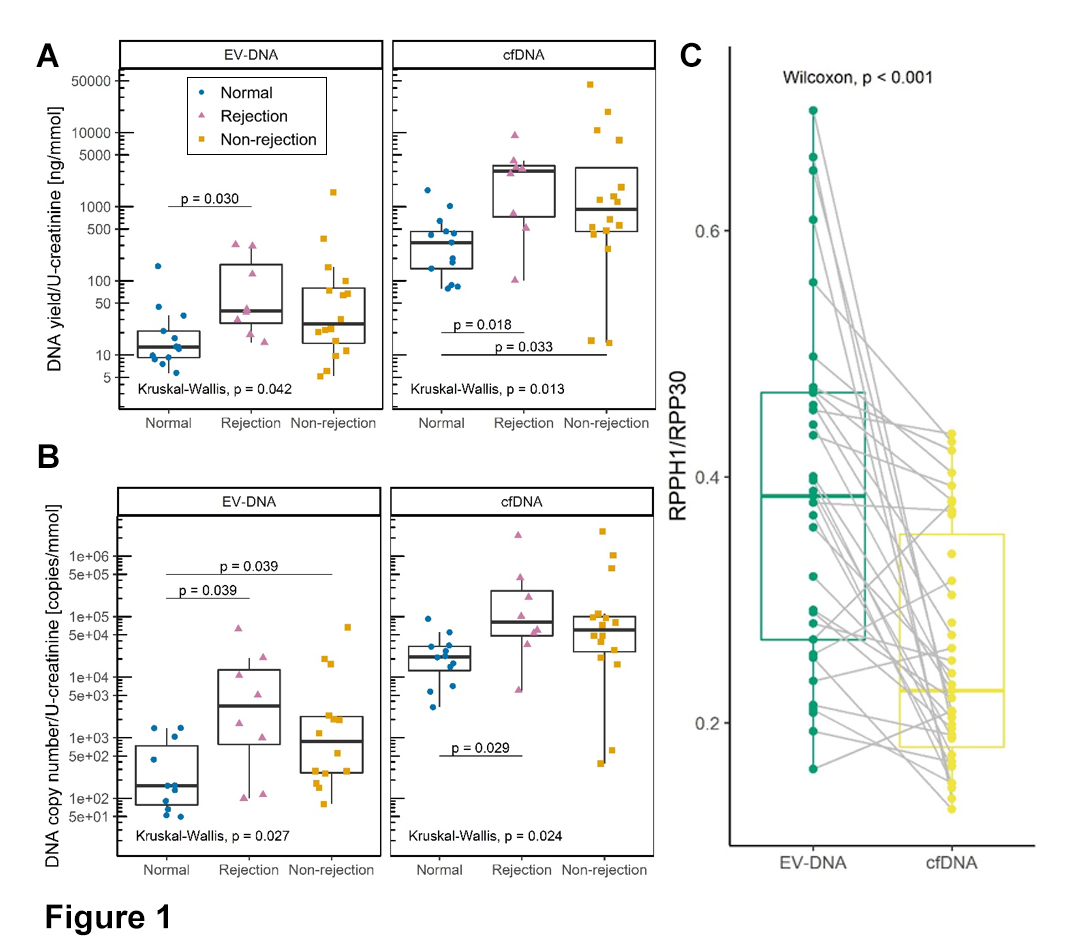
Results: The median uEV concentration was 8.47x1010 / mmol U-creatinine. The concentration was similar in all patient groups, whereas the size parameter differed significantly, as patients with rejection and non-rejection injury had significantly larger uEVs compared with patients with normal histology (177.5 nm and 174.1 nm vs. 160.7 nm, respectively; P=0.045). DNA co-isolated with uEVs and in several parameters correlated with urinary cfDNA. uEV-DNA and cfDNA yields (Figure 1A) and DNA copy number (Figure 1B) were significantly higher in both allograft injury groups. For uEV-DNA, the copy number of donor-derived DNA was significantly higher in patients with ABMR than in patients with TCMR (3044.3 vs. 680.9 copies, respectively; P=0.036). Regarding the long/short gene amplicon ratio, uEV-DNA (green) was less fragmented compared with cfDNA (yellow; Figure 1C). The TEM DNA labelling showed that the DNA was bound to the EV surface. uEV-DNA characteristics correlated with interstitial inflammation, inflammation in areas of fibrosis, and combination of glomerulitis and peritubular capillaritis Banff scores.
Conclusion: In kidney transplant recipients, DNA bound to the surface of uEVs reflects allograft injury, particularly allograft rejection and is therefore a suitable potential biomarker for kidney allograft injury.
Slovenian Research Agency Grants P3-0323, P1-0170, P4-0165, P4-0407.

right-click to download
