
Dr. Rosales is a Post-doctoral Research Associate at the University of Sydney and a Women in Transplantation Sanofi Fellowship Award Winner. She has a growing track record in translational health research in cancer and kidney transplantation. After receiving a Bachelor of Medical Science from The University of Technology, Sydney, Dr. Rosales worked as a Tissue Typing Scientist in the NSW Transplantation and Immunogenetics Services. Following an interest in equity and healthcare delivery, she went on to complete a Master of Public Health at the University of Sydney. Dr. Rosales has worked internationally in cancer clinical trials and recently completed a Ph.D. on the impact of cancer on kidney transplant recipients, including identifying sex and gender differences in outcomes under the supervision of Prof Angela Webster and Prof Kate Wyburn at The University of Sydney. Her Ph.D. projects involved the analysis of linked health administrative and clinical transplantation data to investigate cancer outcomes in kidney transplant recipients and, immunophenotyping kidney transplant recipients with ABMR to identify predictive biomarkers of disease. Her work has informed clinical practice guidelines in keratinocyte cancer management and deceased organ donor acceptance criteria. Dr. Rosales' post-doctoral work aims to bring together her diverse research and project management skills and experience to investigate inequities in health service delivery in cancer and transplantation and create real-world change.
Natural history of CXCR5+ t follicular-like helper (TFH) cells in kidney transplant recipients with rejection
Brenda Rosales1,2, Susan Wan2,3,7, Nicole De La Mata1, Bavani Gunasegaran4, Angela C Webster1,5,6, Helen Mcguire4, Barbara Fazekas4, Kate Wyburn2,7.
1Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia; 2Charles Perkins Centre Renal Research Node, The University of Sydney, Sydney, Australia; 3Department of Renal Medicine, Royal North Shore Hospital, Sydney, Australia; 4Ramaciotti Facility for Human Systems Biology, The University of Sydney, Sydney, Australia; 5Renal Medicine & Research, Westmead Hospital, Sydney, Australia; 6NHRMC Clinical Trials Centre, The University of Sydney, Sydney, Australia; 7Department of Renal Medicine & Transplantation, Royal Prince Alfred Hospital, Sydney, Australia
Introduction: T follicular-like helper cells (Tfh) have been associated with donor-specific antibody (DSA) and antibody-mediated rejection (ABMR) in kidney transplant recipients. However, changes in Tfh according to rejection type are less clear. We sought to describe the natural history of Tfh by rejection type.
Methods: A pilot study of 4 recipients with ABMR were age-sex matched to recipients with TCMR, and recipients with no rejection. Prospectively collected blood samples were analysed retrospectively by cytometry by time of flight and manually gated to identify CD3+CD4+CXCR5+ Tfh (Figure1). Pre-transplant Tfh frequency of CD3+ T cells was compared between rejection groups, pre-transplant DSA and de-novo DSA using student's T-test. Change in Tfh frequency post-transplant was tested between rejection groups using linear mixed regression.
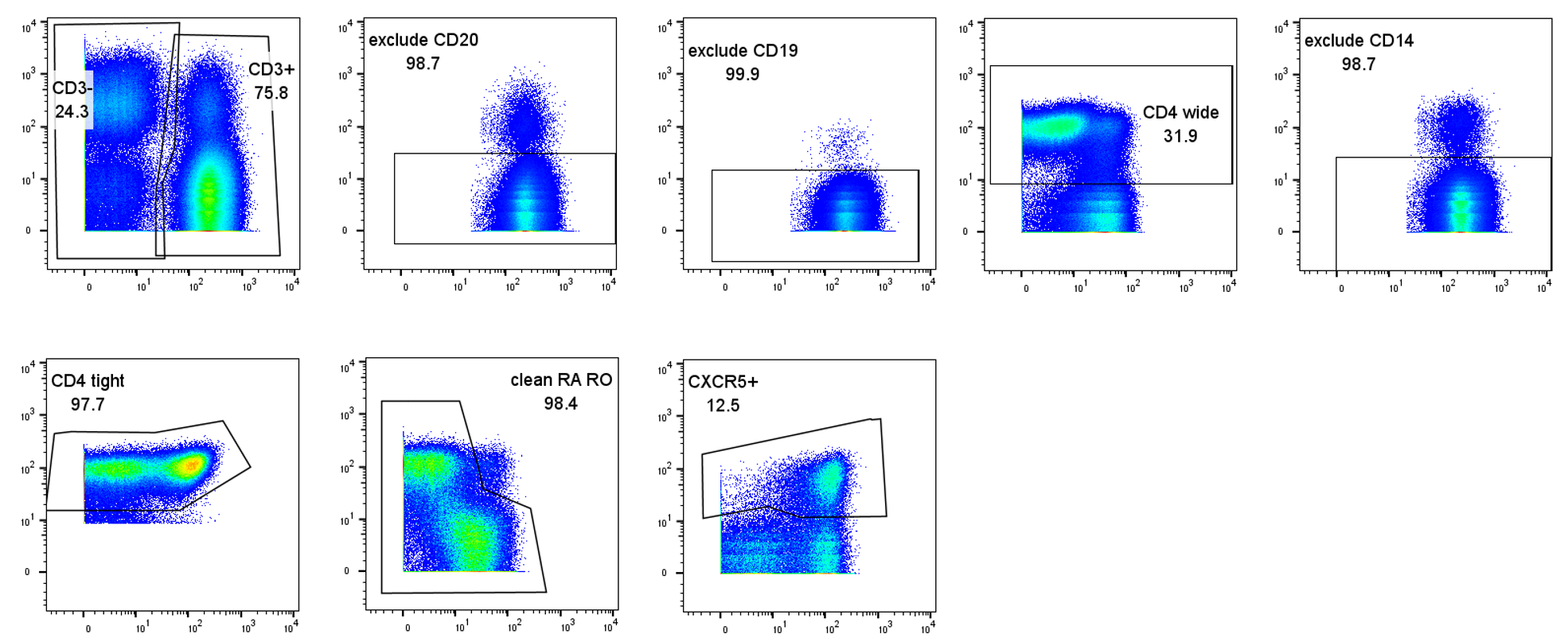
Results: Pre-transplant Tfh frequency was highly variable between individuals (median=3.3%, IQR=1.1-5.9) and not significantly different between rejection groups (p=0.7), those with and without pre-transplant DSA (p=0.4) or those that developed and did not develop de-novo DSA (p=0.7). Early post-transplant Tfh frequency decreased in recipients with subsequent TCMR or stable function but increased in those who developed ABMR (Figure2). Of the four recipients with AMBR, three had a Tfh frequency increase within 60 days prior to ABMR. Change in Tfh frequency over time was 2.7% (95%CI=0.3-5.2) higher in ABMR than stable recipients, adjusting for pre-transplant DSA and de-novo DSA (p<0.001). There was no difference in the change of Tfh frequency between TCMR and stable recipients.
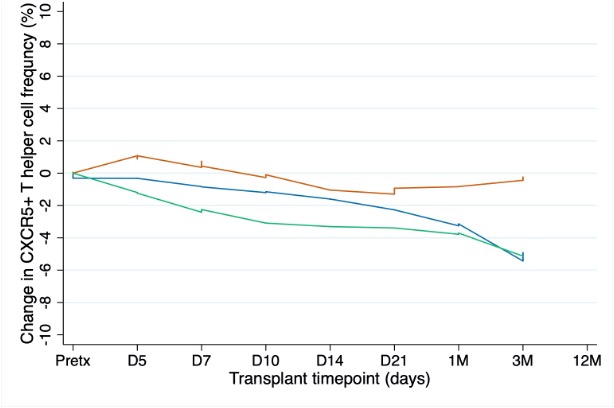
Conclusion: In the early post-transplant period and immediately before rejection, Tfh frequency increased above pre-transplant levels in patients who subsequently developed ABMR. This was not observed in stable patients or those with TCMR. These results will inform timepoints appropriate for screening in larger cohort studies.
Kidney Health Australia. Ramaciotti Facility for Human Systems Biology. RPAH Transplant Insititute.

right-click to download
