Dynamic of donor derived cell‐free DNA and blood gene expression after pancreas transplantation
Maria J Ramirez-Bajo1, Jordi Rovira1, Elisenda Banon-Maneus 1, Natalia Hierro-Garcia 1, Marta Lazo 1, Maria A Garcia-Criado 2, Marta Donas3, Maria Gomez6, Joana Ferrer 4, Enric Esmatjes 5, David Cucchiari 1,7, Nuria Esforzado 7, Ignacio Revuelta 1,7, Gaston Piñeiro 1,7, Vicens Torregrosa 7, Federic Cofan 7, Josep M Campistol 1,7, Fritz Diekmann 1,7, Pedro Ventura-Aguiar 1,7.
1Laboratori Experimental de Nefrologia i Trasplantament, Fundacio Clinic - IDIBAPS, Barcelona, Spain; 2Radiology Department, Hospital Clinic de Barcelona, Barcelona, Spain; 3Eurofins Megalab, Madrid, Spain; 4Hepatobiliopancreatic Surgery Department, Hospital Clinic de Barcelona, Barcelona, Spain; 5Endocrinology Department, Hospital Clinic de Barcelona, Barcelona, Spain; 6Eurofins Transplant Genomic , Framingham , MA, United States; 7Nephrology and Kidney Transplant Department, Hospital Clinic de Barcelona, Barcelona, Spain
Introduction: Donor-derived cell-free DNA (dd-cfDNA) is a noninvasive test that had demonstrated high predictive performance for acute rejection in solid organ transplant. However, combining dd-cfDNA with a gene expression profile assay could improve the diagnostic of subclinical acute rejection episodes. We aimed at evaluating the combination of these two molecular diagnostic methods in pancreas transplantation.
Materials and Methods: We conducted a prospective longitudinal study including all pancreas transplant recipients from our center from January 2017 to December 2018. Plasma samples were collected before transplant (D0), and at 1h, 24h and 7 days (D7) post-transplant, and at time of pancreas biopsy – either surveillance at 3 weeks (B3) and 12 months (B12), or per clinical indication. Biopsies were classified according to the Banff criteria. Dd-cfDNA percentage (%) was assessed using Eurofins Genoma AlloNext® by Next Generation Sequencing (NGS). Gene expression profiles, measured at the time of pancreas biopsy, were analysed with TruGraf®, a Blood Gene Expression Test of Eurofins_Transplant Genomics that uses a PCR-based assay to analyse gene expression from peripheral blood and assign a result of either “TX” (normal, no rejection) or “not-“TX (rejection).
Results: A total of 77 patients were included (SPK n=65; PAK n=12). Dd-cfDNA increased significantly at 1h and 24h after transplantation compared to baseline (D0), most likely reflecting ischemia/reperfusion damage. No correlation with either donor demographics nor cold ischemia time was identified (p>0.05). In 52 cases a biopsy-related sample was obtained (Banff criteria: No rejection n=33; Indeterminate n=5; TCMR grade 1-3 n=15; ABMR n=4). In patients with stable graft function (no rejection during the first 12months) dd-cfDNA decreased by D7 and remained low at B3 and B12 (D0 (0.27±0.14), 1h (4.30±2.53), 24h (2.41±1.79), D7 (0.65±0.47), B3 (0.47±0.28) and B12 (0.61±1.31)) Figure 1.
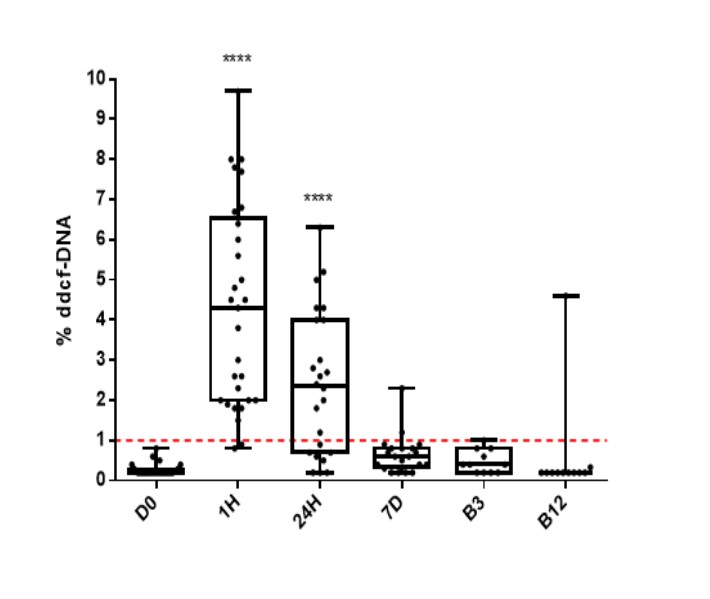
However, in patients with biopsy proven acute rejection (TCMR; 1.74±2.08 or ABMR; 4.42±2.93; p<0.01) % dd-cfDNA increased compared with no proven acute rejection biopsies (N-BPAR; 0.45±0.77). Furthermore, a 1% threshold had a positive predictive value to detect 86% rejection, whereas the negative predictive value was 69%. In the gene expression study 75 patients with biopsy-related sample were included: N-BPAR=41; TCMR=23; ABMR=3 and undetermined=8. The study is currently ongoing. We expect to have more conclusive datain the coming months..
Conclusion: We describe the first dd-cfDNA dynamic analysis in pancreas transplant recipients, validating its application for longitudinal monitoring in clinical practice. Combining these results with gene expression profile assay may improve the performance for the diagnosis of subclinical rejection.

right-click to download
