Donor-derived cell-free DNA surveillance level kinetics after lung transplantation: experience from a single-center feasibility study
Pascal Pedini1,2, Benjamin Coiffard3, Nicem Cherouat1, Silvia Casas4, Jacques Chiaroni1,2, Coralie Frassati1,2, Martine Reynaud Gaubert3, Christophe Picard1,2.
1Immunogenetics Laboratory, Etablissement Français du Sang, Marseille, France; 2ADES UMR 7268, Aix Marseille Univ, Marseille, France; 3Lung transplant department, APHM, Marseille, France; 4CareDx, Stockholm, Sweden
Introduction: Previous longitudinal observational studies have described increases of donor-derived cell-free DNA (ddcfDNA) in lung transplant acute rejection and infection, supporting its use as a potential non-invasive marker for surveillance monitoring. Here we present the preliminary data of a feasibility study for implementing ddcfDNA in routine clinical care at our lung transplant center with test performed in house.
Method: Single-center observational study including 45 newly adult lung transplant patients (> 18 years old) followed at the CHU Hôpital Nord (Marseille, France). Subjects were monitored prospectively and plasma ddcfDNA levels tested serially at day 15, 30, 90 and 180 post-transplant with AlloSeq cfDNA assay (CareDx), and with protocol transbronchial biopsy at day 30 and at day 15 according to the symptoms.
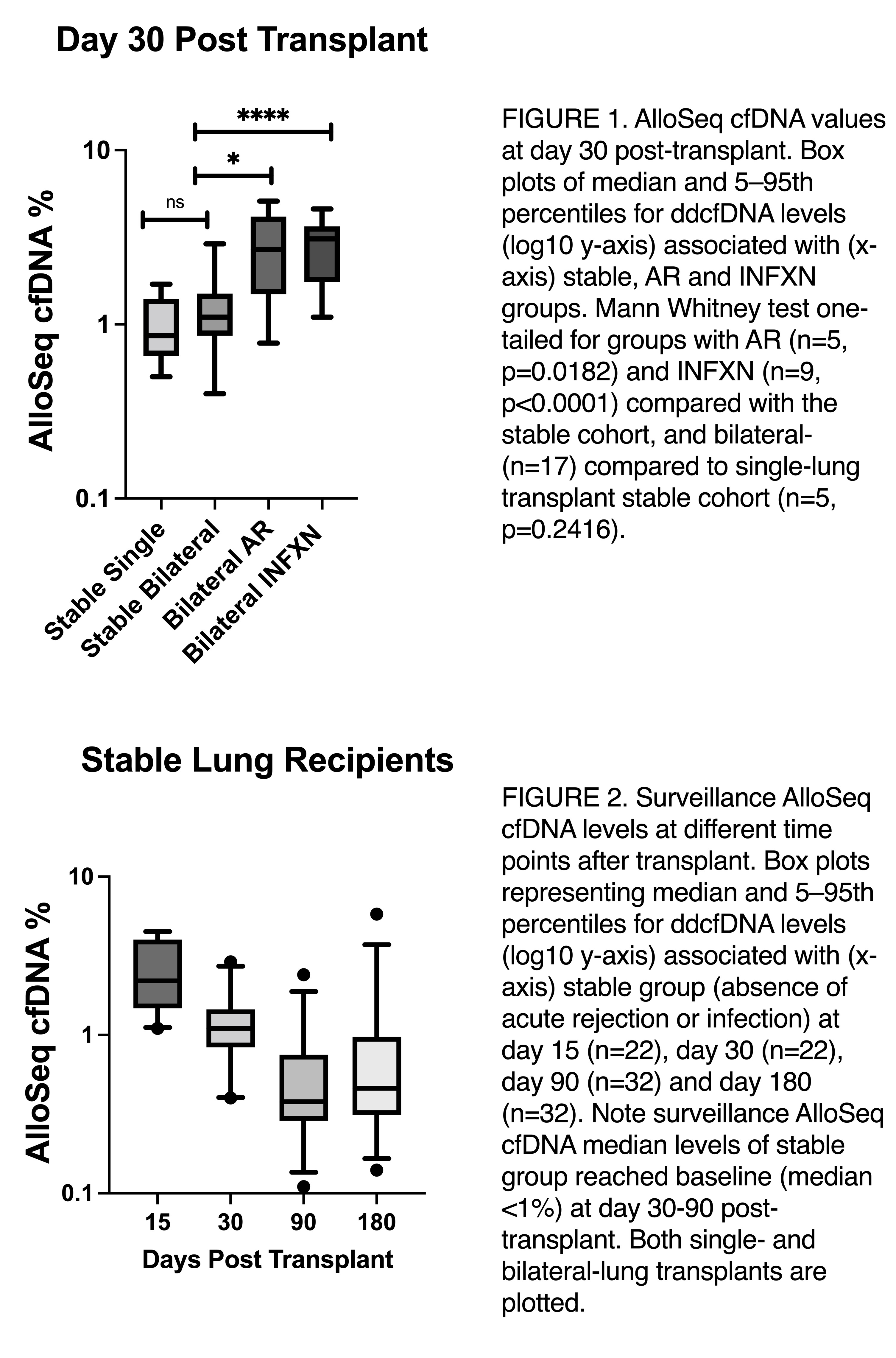
Results: The cohort of 45 study subjects had an average age at transplantation of 52.7 years, 53% were female and 87% received bilateral lung transplants. At day 15, all patients had a surveillance level >1%, 8/45 (18%) had biopsy proven acute rejection (AR) and 11/45 (24%) had evidence of infection (INFXN). Although at day 15 median levels of ddcfDNA in stable patients (absence of acute rejection or infection) were higher for bilateral- than for single-lung transplantation (17, 3.6% vs. 5, 1.4%, p=0.0054), the ddcfDNA decline kinetics let to no differences at day 30 (17, 1.1% vs. 5, 0.86%, p=0.2416). This initial ddcfDNA decay was yet not enough to allow lung recipients without acute rejection or infection to reach baseline levels, as 14/252 (64%) still had values >1%. At day 30, 5/45 (11%) patients had biopsy proven acute rejection and 9/45 (20%) had infection. The median ddcfDNA level for groups with acute rejection (5, 2.7%) or infection (9, 3.1%) was significantly elevated compared with the stable group (p=0.0182 and p<0.0001, respectively). At day 90 and 180, majority of subjects had surveillance ddcfDNA values <1% cut-off, and higher values were only seen in 2/45 (4%) and 8/45 (18%) of the cases, respectively.
Conclusion: Measurement of ddcfDNA in the early post-transplantation period showed decline kinetics at day 30 compared to day 15, reaching stable baseline at day 90. Although discrimination between rejection or infection in contrast to stable allograft was already observed at day 30, dd-cfDNA surveillance may be a clinically useful tool for the assessment of lung allograft function and rule out of injury when patient achieves baseline, which in our cohort observed to happen between day 30 and 90.

right-click to download
