
Spatial transcriptomic profiling reveals immunosuppression and immunosenescence-driven skewing of immune response in cutaneous squamous cell carcinoma immune response in kidney transplant recipients, associating with adverse cancer-related outcomes
Matthew Bottomley1,2, Eleni Ieremia3, Paul N. Harden1, Kathryn J. Wood2, Joanna Hester2, Fadi Issa2.
1Oxford Kidney Unit, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom; 2Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 3Department of Cellular Pathology, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
Introduction: Cutaneous squamous cell carcinoma (SCC) is the most common malignancy in immunosuppressed populations, demonstrating increased aggression compared to the general population. One such immunosuppressed group is organ transplant recipients. We previously demonstrated peripheral blood markers of immune ageing (immunosenescence) identifies kidney transplant recipients (KTR) with poorer cancer-related outcomes, with earlier SCC recurrence and greater risk of metastatic SCC/non-keratinocyte malignancy development which remains significant after multivariate correction. We sought to explore the mechanism underlying this association.
Methods: Excised SCCs from KTR (n=16) and non-immunosuppressed controls (NIC, n=11) were assessed by immunohistochemistry to quantify infiltrating T cell populations. High-resolution spatial transcriptomic profiling was utilised to evaluate alterations in gene expression and transcriptomic pathways (using gene set enrichment analysis using the Reactome knowledgebase) within the tumour and immune-rich peritumoural regions of SCC excised from KTR using the 1800-gene ‘Cancer Transcriptome Atlas’ on the Nanostring GeoMx platform (n=6).
Results: CD8+ and FOXP3+ tumoural T cell density decreased with advancing peripheral blood immunosenescence in KTR but not in NIC on immunohistochemistry. Across the KTR cohort, upregulation of pathways in the peritumoural infiltrate relating to TCR signalling, co-stimulation, chemotaxis and immunoregulation was seen compared to the tumour bed (Figure 1A). Within immunosenescent KTR, there was greater enrichment of pathways relating to chemotactic signalling and phagocytosis were seen (Figure 1B). Cellular deconvolution revealed enhanced accumulation of macrophages with an M2 gene signature, associating with reduced monocytic dendritic cell and CD8+ T cell density (Figure 1C).
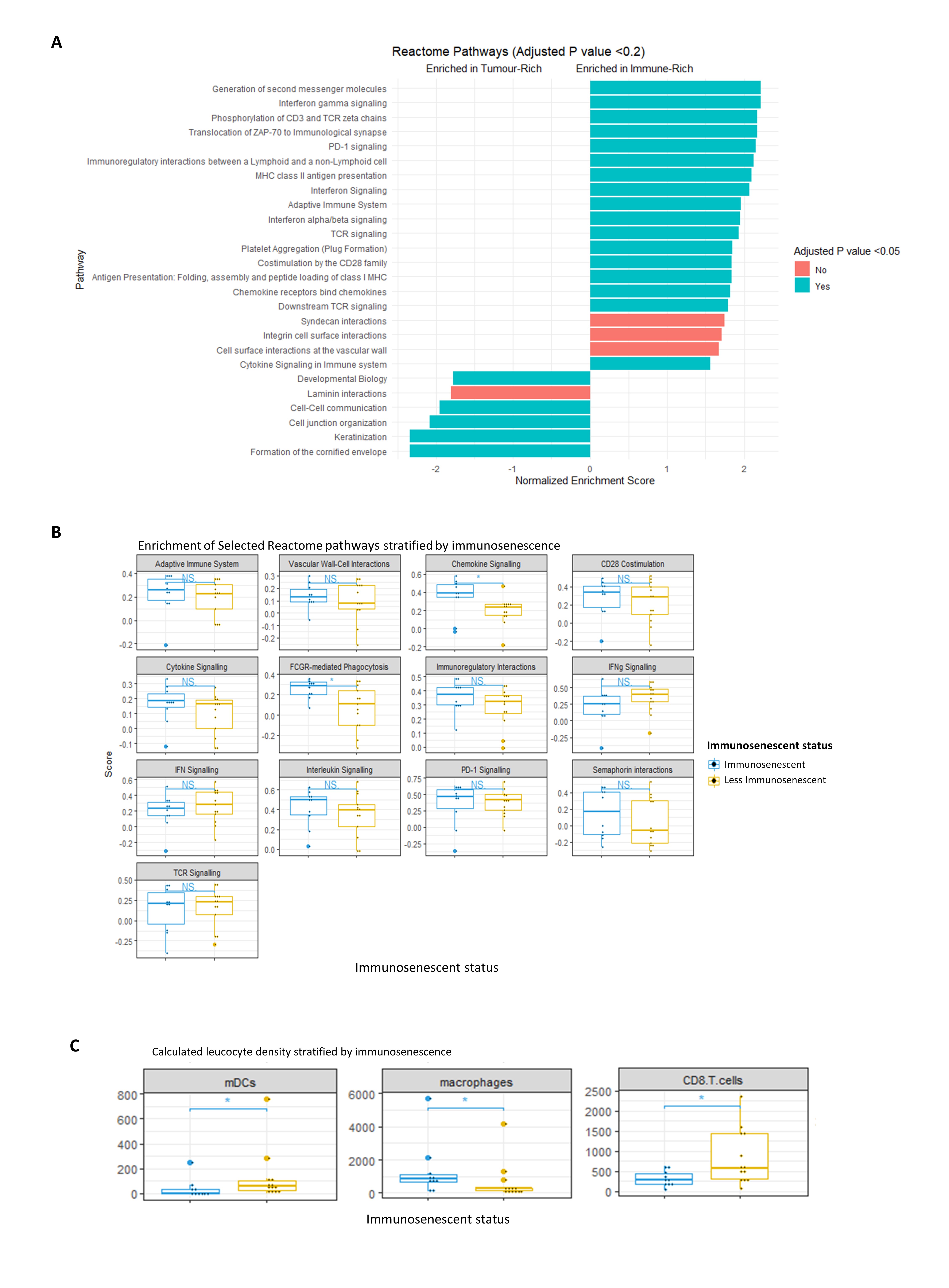
Conclusion: We conclude that immunosenescence synergises with iatrogenic immunosuppression in cutaneous malignancy, leading to skewing of the immune milieu culminating in accumulation of suppressive M2 macrophages and associating with adverse cancer-related outcomes. Quantification of immunosenescence in KTR may permit risk stratification facilitating potential preventative interventions.
The work presented here was supported by grants from the Wellcome Trust, British Skin Foundation, Oxford Hospital Charities and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Science (CIFMS), China (grant number: 2018-I2M-2-002).

right-click to download
