Gwangmin Lee, Korea has been granted the TTS-KST International Transplantation Science Mentee-Mentor Awards
Anti-C4d chimeric antigen receptor regulatory T cells suppressed allograft rejection in ABO-incompatible heart transplantation
Gwangmin Lee1,2, Sun-Kyung Lee1,2, Joon Young Jang2, Honglin Piao1,2, Dong Kyu Han1,2, Ji-Jing Yan2, Lori J. West3, Peter J. Cowan4, Jaeseok Yang2.
1Department of Medicine, Graduate School, Seoul National University College of Medicine, Seoul, Korea; 2Division of Nephrology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea; 3Department of Pediatrics, Surgery and Immunology, Alberta Transplant Institute, Canadian National Transplant Research Program, University of Alberta, Edmonton, AB, Canada; 4Immunology Research Centre, St. Vincent's Hospital Melbourne, Melbourne, Australia
Introduction: Antibody-mediated rejection (ABMR) is the main hurdle in ABO blood group-incompatible (ABOi) transplantation. C4d deposition is a marker of ABMR and is also found in most ABOi allograft tissues as a result of accommodation. Previously, we reported that anti-C4d chimeric antigen receptor (CAR) regulatory T cells (Tregs) suppressed ABMR in ABOi allografts. Here, we aimed to improve efficacy of anti-C4d CAR Tregs by modifying CAR structure and increasing dose of CAR Tregs.
Methods: Intracellular costimulatory domains of anti-C4d CAR Treg consisted of mouse CD3ζ and CD28. CD62L+CD4+CD25+ T cells were sorted and transduced with retroviral CAR using retronection on two consecutive days. We assessed in vitro suppressive function of anti-C4d CAR Tregs against T cell proliferation in response to polyclonal stimulation. Wild-type C57BL/6J mice were sensitized on day -21 and on day -14 by injecting human blood group A-expressing cells. Hearts from human blood group A-transgenic BALB/c mice were transplanted into the sensitized CD45.2+ C57BL/6J mice to make an anti-ABO antibody-mediated rejection model. CD45.1+ non-transduced, control CAR, or anti-C4d CAR Tregs (1×106) were transferred into recipient mice on day-1 and day 2 after transplantation in combination with prednisolone, tacrolimus, and rapamycin. ABOi heart allograft were analyzed for tissue damage, cellular infiltration, and cytokine expression on day 7.
Results: Anti-C4d CAR Tregs express Foxp3, CD25, CTLA-4, LAP, and GITR that are associated with immunosuppressive functions of Tregs, to similar extent as either nontransduced or control CAR Tregs. In vitro suppressive activity of anti-C4d CAR Tregs was also similar as that of nontransduced Tregs and control CAR Tregs. ABOi heart allografts showed typical features of ABMR, such as, peritubular capillitis and diffuse endothelial C4d+ deposition. Adoptive transfer of anti-C4d CAR Tregs suppressed ABMR-related tissue injury and significantly prolonged ABOi heart allograft survival compared to PBS control group, nontransduced Treg group, and control CAR Treg group. Both flow cytometric analysis and immunofluorescence imaging study demonstrated that number of CD45.1+Foxp3+ Treg infiltration into heart allograft were significantly higher in anti-C4d CAR Treg group compared to PBS control, nontransduced CAR Treg group, and control CAR Treg group. Expression of IL-2, TNF-α, and IFN-γ in heart allografts was significantly lower in anti-C4d CAR Treg group than PBS control group.
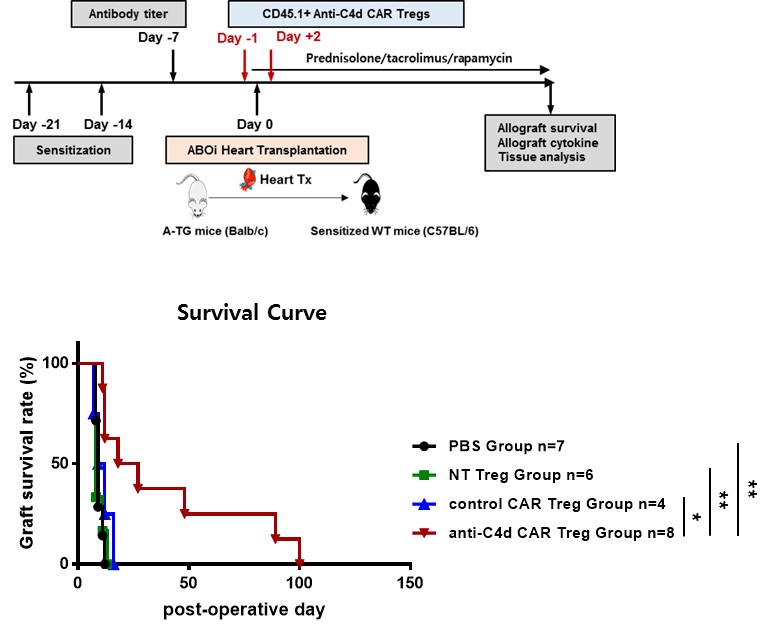
Conclusions: Anti-C4d CAR Tregs infiltrated into ABOi heart allograft with diffuse C4d deposition to more extent and thereby suppressed ABMR in ABOi heart allografts more effectively, compared to nontransduced Tregs and control CAR Tregs.
Keywords: ABO-incompatible transplantation; C4d; Chimeric antigen receptor; Regulatory T cells; Rejection.

right-click to download
