Selective Bcl-2 inhibition promotes hematopoietic chimerism and allograft tolerance without myelosuppressive complications
Takayuki Hirose1, Hajime Sasaki1, Tetsu Oura1, David Ma1, Grace Lassiter1, Abbas Dehnadi1, Isabel Hanekamp1, Ivy Rosales2, Robert B. Colvin2, Benedict A. Coisimi1, Pietro Cippa3, Thomas Fehr4, Tatsuo Kawai1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2Pathology, Massachusetts General Hospital, Boston, MA, United States; 3Division of Nephrology, Ente Ospedaliero Cantonale, Lugano, Switzerland; 4Department of Internal Medicine, Cantonal Hospital Graubuenden, Chur, Switzerland
Introduction: Hematopoietic stem cell transplantation (HSCT) has been an only approach that reproducibly achieved renal allograft tolerance in humans. However, severe myelosuppression and toxicities after myeloablative conditioning have hampered wider clinical application. To achieve hematopoietic stem cell (HSC) engraftment, it is essential to make space in the bone marrow (BM) niches by depleting host HSCs. This has been achievable only by nonselective treatments such as irradiation or chemotherapeutic drugs. Therefore, a novel approach more selectively capable of depleting HSCs is needed to widen the clinical application of HSCT. Based on murine studies that showed successful induction of mixed chimerism and allograft tolerance without irradiation by Bcl-2 inhibition, we evaluated an FDA approved Bcl-2 inhibitor (Venetoclax, Vntx) in our mixed chimerism inducing conditioning regimen.
Methods: Cynomolgus monkeys received kidney and bone marrow transplantation after conditioned with various nonmyeloablative regimens which included total body irradiation (TBI), thymic irradiation (TI) and anti-thymocyte globulin (ATG), followed by a short post-transplant course of costimulatory blockade (CoB) and Cyclosporine. The study groups were consisted of 3Gy TBI, no Vntx (Group A), 1.5Gy TBI, no Vntx (Group B), TBI 1.5Gy with Vntx(10 mg/kg x 11 on days -4 to 6) (Group C), TBI 1.5Gy with Vntx but Belatacept as CoB (Group D), no TBI with Vntx (Group E), no TI with Vntx (Group F), no CoB with Vntx (Group G).
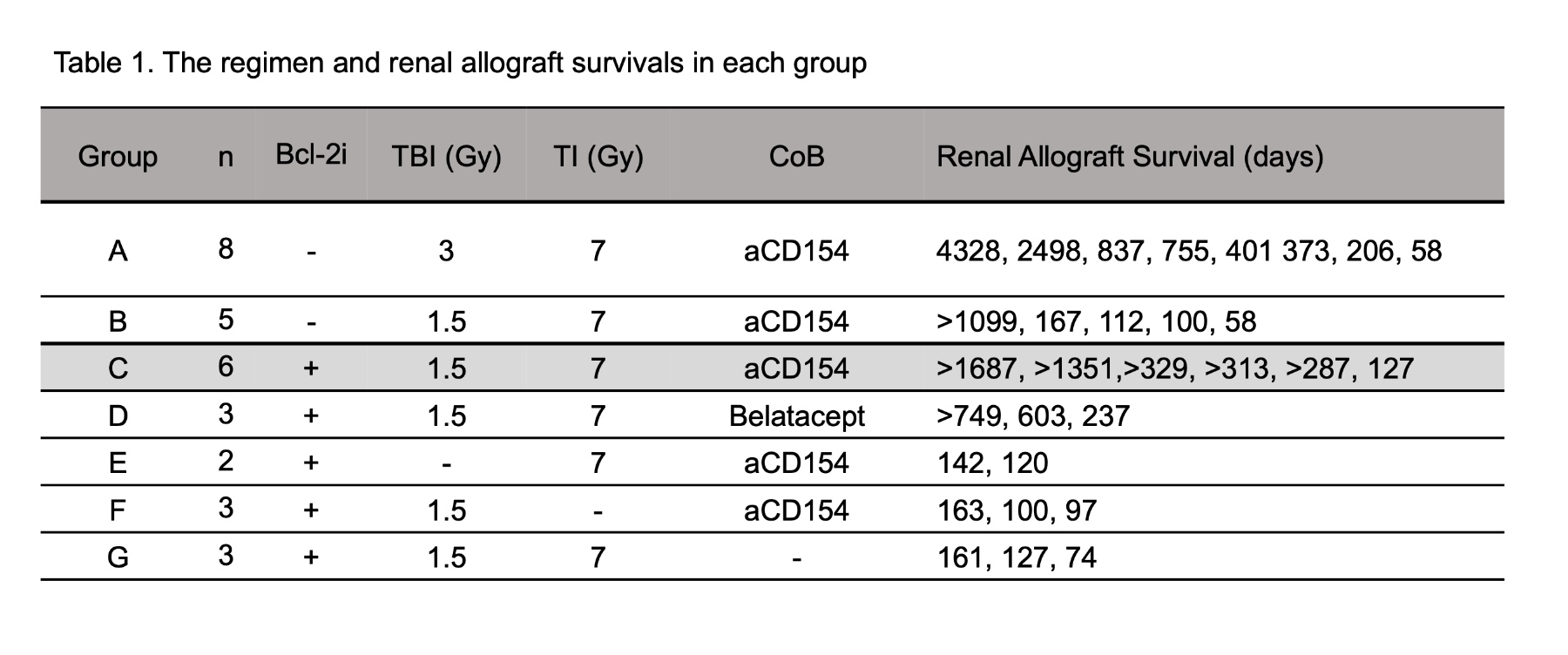
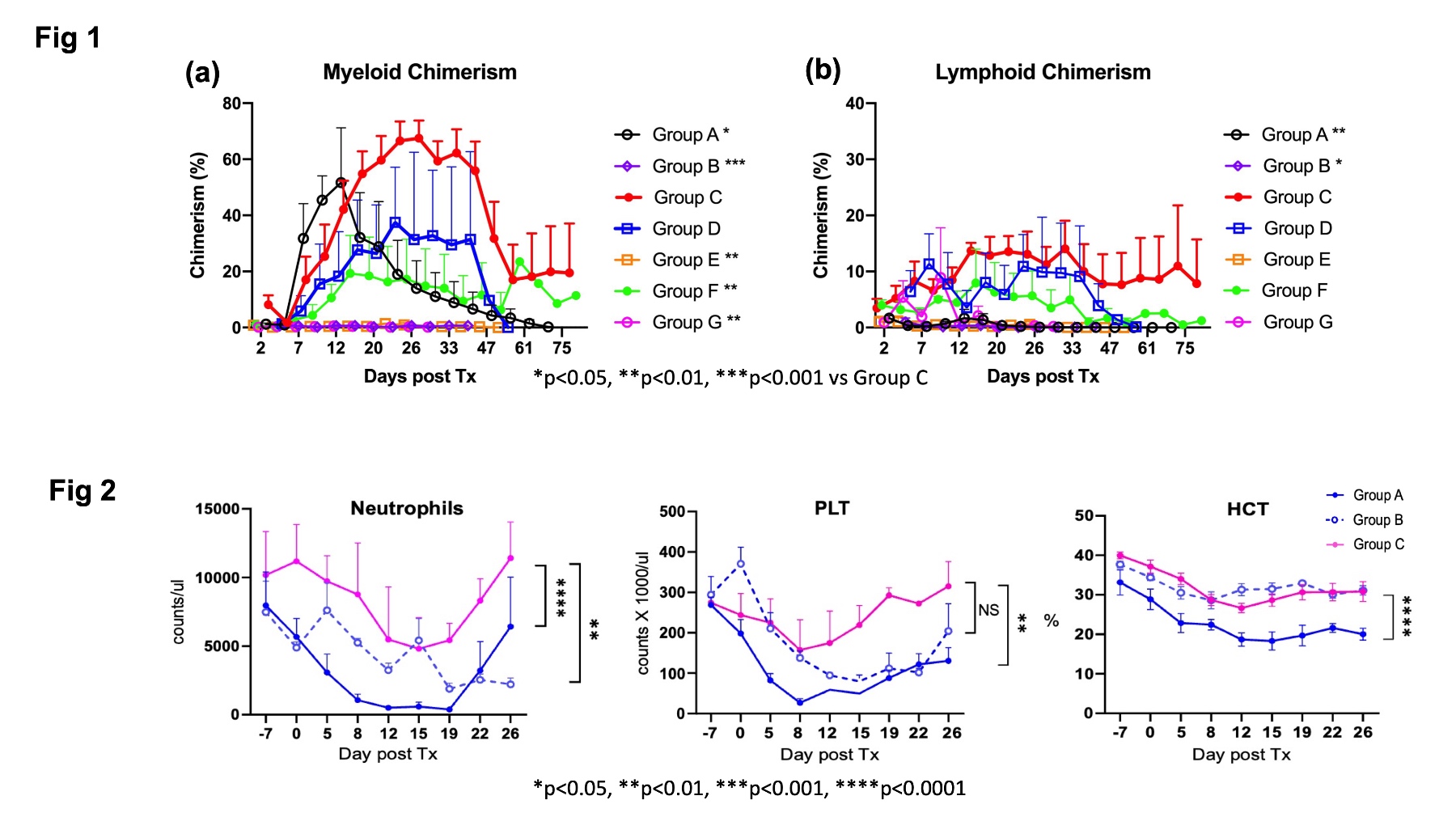
Results: Most recipients treated with 3Gy TBI (Group A) developed mixed chimerism and long-term immunosuppression (I.S.)-free renal allograft survival (Table 1), but suffered from transient but severe pancytopenia. With reduced TBI (1.5Gy), all recipients failed to develop chimerism, and 4/5 developed acute rejection. By adding Vntx, despite the dose of TBI being reduced to half (1.5Gy), all six recipients (Group C) developed significantly superior chimerism (Figure 1) than regimens without Bcl-2 inhibition (Groups A and B), and 5/6 achieved long-term I.S.-free allograft without pancytopenia (Figure 2). With Belatacept in place of anti-CD154 mAb, 2/3 achieved chimerism and long-term I.S. free allograft survival. The current studies also revealed that a minimal non-toxic dose of TBI, TI and CoB remain to be essential for long-term I.S.-free renal allograft survival. Without TBI (Group E) or CoB (Group G), the recipients failed to develop chimerism and rejected allografts despite administration of Vntx. Without TI (Group F), all recipients developed mixed chimerism but failed to achieve tolerance.
Conclusion: Bcl-2 inhibition with Vntx significantly promoted mixed chimerism and renal allograft tolerance without myelosuppressive complications. A minimal non-toxic dose of TBI, TI, and CoB were still required for chimerism and allograft tolerance induction even with Bcl-2 inhibition.

right-click to download
